30-second summary
Electric Battery
An electric battery is essentially a source of DC electrical energy. It converts stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. A typical battery consists of one or more voltaic cells.
Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals.
Even though a wide range of types of batteries exists with different combinations of materials, all of them use the same principle of the oxidation-reduction reaction.

An electric battery is essentially a source of DC electrical energy. It converts stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. A typical battery consists of one or more voltaic cells.
The fundamental principle in an electrochemical cell is spontaneous redox reactions in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated.
Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals. Even though a wide range of types of batteries exists with different combinations of materials, all of them use the same principle of the oxidation-reduction reaction. Batteries are designed so that the energetically favorable redox reaction can occur only when electrons move through the external part of the circuit.
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction. Because most of the resulting voltages are around 2V, cells are connected in series to obtain more practical electrical potentials (i.e. six 2V lead acid cells are connected in series to obtain a typical 12V battery).
Batteries are made of an extensive range of materials resulting in different capabilities and behaviors in the functionality of the battery. The most common ones are lead, nickel, and lithium, each of them with different outputs and specific for some different purposes depending on the requirements.
Chemistry of Electric Batteries
In simple terms, each battery is designed to keep the cathode and anode separated to prevent a reaction. The stored electrons will only flow when the circuit is closed. This happens when the battery is placed in a device and the device is turned on.
When the circuit is closed, the stronger attraction for the electrons by the cathode (e.g. manganese dioxide in alkaline batteries) will pull the electrons from the anode (e.g. zinc) through the wire in the circuit to the cathode electrode. This battery chemical reaction, this flow of electrons through the wire, is electricity.
If we go into detail, batteries convert chemical energy directly to electrical energy. Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals.
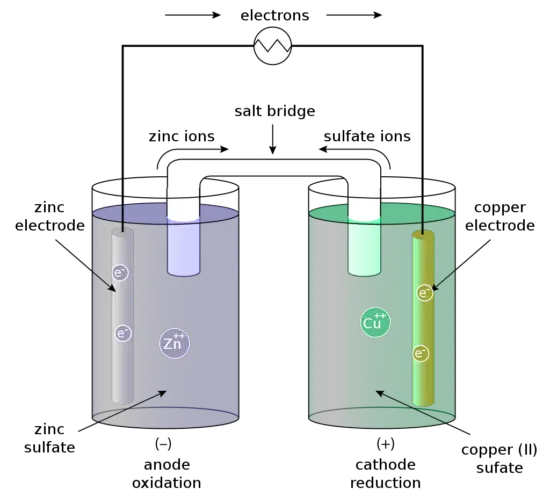
Even though a wide range of types of batteries exists with different combinations of materials, all of them use the same principle of the oxidation-reduction reaction. In an electrochemical cell, spontaneous redox reactions take place in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated. The redox reaction is a chemical reaction that produces a change in the oxidation states of the atoms involved. Electrons are transferred from one element to another. As a result, the donor element, which is the anode, is oxidized (loses electrons) and the receiver element, the cathode, is reduced (gains electrons).
For example, the Daniell cell consists of two electrodes of dissimilar metals, Zn and Cu; each electrode is in contact with a solution of its own ion; Zinc sulfate and copper sulfate respectively. The redox reaction that occurs in the Daniell Cell is:
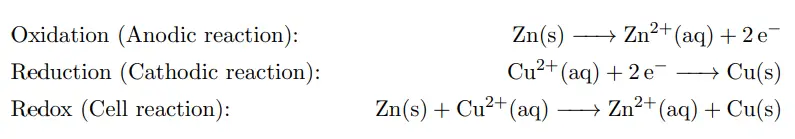
These processes result in the accumulation of solid copper at the cathode and the corrosion of the zinc electrode into the solution as zinc cations. Excess electrons produced by the oxidation of zinc metal are “pushed” out of the anode, which is, therefore, the negative electrode, travel through the wire and are “pulled” into the copper cathode where they are consumed by the reduction of copper ions. The electrical current will cause a potential difference and an electromotive force. The spontaneous reaction will occur because zinc is a better reducing agent than copper and thereby more able to emit electrons. A stronger ability to emit electrons at the anode will cause a stronger electromotive force. A salt bridge or ion bridge, in electrochemistry, is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell. It maintains electrical neutrality within the internal circuit.
Composition of Battery
The alkaline battery consists of five parts:
- Inner current collector (pin)
- Anode. The active material in the anode is Zn. With a standard electrode potential (SEP) of −0.76 volts, zinc is used as an anode material for batteries. (More reactive lithium (SEP −3.04 V) is used for anodes in lithium batteries ).
- Separator. A separator is a permeable membrane placed between a battery’s anode and cathode. For example, non-woven, fibrous fabric that separates the electrodes.
- Cathode. The active material of the cathode is manganese dioxide. The principal use for MnO2 is for dry-cell batteries, such as the alkaline battery and the zinc-carbon battery.
- Electrolyte. Aqueous potassium hydroxide is employed as the electrolyte in alkaline batteries.
- Outer current collector (can)
[alkaline-battery]
Characteristics of Electric Batteries
To compare and understand the capability of each battery, some important parameters are characteristic of each battery, also within a type of battery. These parameters are a reference when a battery is needed, and specific qualities are required since batteries are used in all types of devices and for infinite purposes.
Cell Voltage
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
Cut-off Voltage
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
Capacity
The coulometric capacity is the total Amp-hours available when the battery is discharged at a certain discharge current from 100% SOC to the cut-off voltage.
C-rate of Battery
C-rate is used to express how fast a battery is discharged or charged relative to its maximum capacity. It has units h−1. A 1C rate means that the discharge current will discharge the entire battery in 1 hour.
Self-discharge
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied.
Degradation
Some degradation of rechargeable batteries occurs on each charge–discharge cycle. Degradation usually occurs because electrolyte migrates away from the electrodes or because active material detaches from the electrodes.
Depth of Discharge
Depth of discharge is a measure of how much energy has been withdrawn from a battery and is expressed as a percentage of full capacity. For example, a 100 Ah battery from which 40 Ah has been withdrawn has undergone a 40% depth of discharge (DOD).
State of Charge
The state of charge refers to the amount of charge in a battery relative to its predefined “full” and “empty” states i.e. the amount of charge in Amp-hours left in the battery.
Types of Batteries
The following list is a summary of notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery
Frequently asked questions
A primary battery or primary cell is a non-rechargeable battery that is designed to be used once discarded after use. This means that the redox reaction within the cell is not reversible like in a secondary (rechargeable) battery. Secondary batteries, also known as secondary cells, or rechargeable batteries, are batteries, that can be recharged by driving electric current in the opposite direction of the discharge current.
In practice, the alkaline batteries and the rechargeable batteries can be used interchangeably in sets. They have only different voltage characteristics. It is given by their different chemistry. Primary cells gradually drop in voltage from use, they start at 1.5 volts, drop to 1.2 and continue to 1.0 where the appliance stops working. Secondary cells operate more uniformly even with only 1.2 volts, they have flat discharge where they pretty much stay at 1.2 volts until depleted and then drop off very quickly to below 1.0 volts.
Alkaline batteries are prone to leaking potassium hydroxide (KOH), a caustic agent that can cause respiratory, eye and skin irritation. The reason for leaks is that as batteries discharge — either through usage or gradual self-discharge — the chemistry of the cells changes and some hydrogen gas is generated. The outer casing of the battery prevents the hydrogen gas from leaking. However, if a battery is left unused in a device for an extended period, the resulting gas buildup can rupture the casing and cause leakage.









