30-second summary
Cell Voltage
The cell voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
The overall voltage of electric batteries is determined by:
- Chemistry. The potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
- Number of cells. Batteries in series produce a voltage equal to the number of batteries multiplied by the voltage of each individual battery.
Different battery chemistry creates different cell voltage.
- 1.5V (DC) – A common open circuit voltage for non-rechargeable alkaline batteries (e.g. AAA, AA and C cells).
- 3V (DC) – Lithium-based primary cells are batteries that have metallic lithium as an anode. The voltage of most lithium-metal cells (e.g. button cells) is 3V.
- 3.8V (DC) – Almost all lithium-ion batteries work at 3.8 volts. In order to make current flow from the charger to the battery, there must be a potential difference. Therefore battery chargers or USBs for almost all smartphones provide a voltage of 5V.
- 12V (DC) – A common voltage for automobile batteries is 12 volts (DC). But this battery consists of six 2V lead cells.
An electric battery is essentially a source of DC electrical energy. It converts stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. A typical battery consists of one or more voltaic cells.
The fundamental principle in an electrochemical cell is spontaneous redox reactions in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated.
Cell Voltage

The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
The voltage produced by each lithium-ion cell is about 3.6 volts. This has many advantages. Being higher than that of the standard nickel-cadmium, nickel metal hydride, and even standard alkaline cells at around 1.5 volts and lead acid at around 2 volts per cell, the voltage of each lithium-ion cell is higher, requiring fewer cells in many battery applications.
Because most of the resulting voltages are around 2V, cells are connected in series to obtain more practical electrical potentials (i.e. 2V lead acid cells are connected in series to obtain a typical 12V battery).
To know the voltage of a battery, batteries are marked with nominal voltages which is the average voltage a cell outputs when is fully charged, but this may differ from the open circuit voltage.
In addition, some factors like low temperature can decrease the expected voltage output and increase with higher temperature, which is favorable for the electrochemical reactions.
To avoid batteries to discharge below a certain level which could cause damaging the battery, there is a voltage limit called cut-off voltage.
- 1.5V (DC) – A common open circuit voltage for non-rechargeable alkaline batteries (e.g. AAA, AA and C cells).
- 3V (DC) – Lithium-based primary cells are batteries that have metallic lithium as an anode. The voltage of most lithium-metal cells (e.g. button cells) is 3V.
- 3.8V (DC) – Almost all lithium-ion batteries work at 3.8 volts. In order to make current flow from the charger to the battery, there must be a potential difference. Therefore battery chargers or USBs for almost all smartphones provide a voltage of 5V.
- 12V (DC) – A common voltage for automobile batteries is 12 volts (DC). But this battery consists of six 2V lead cells.
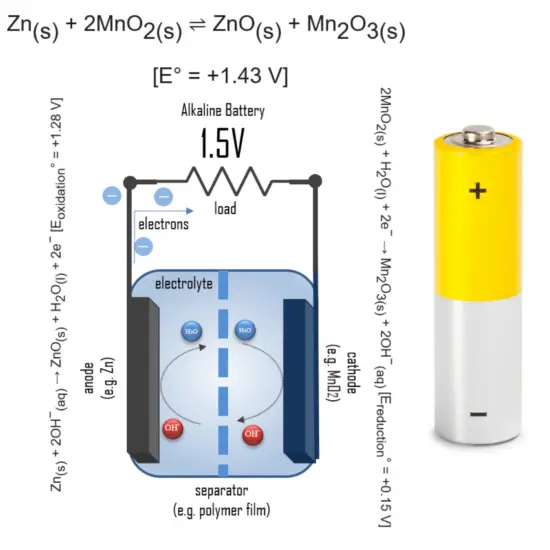
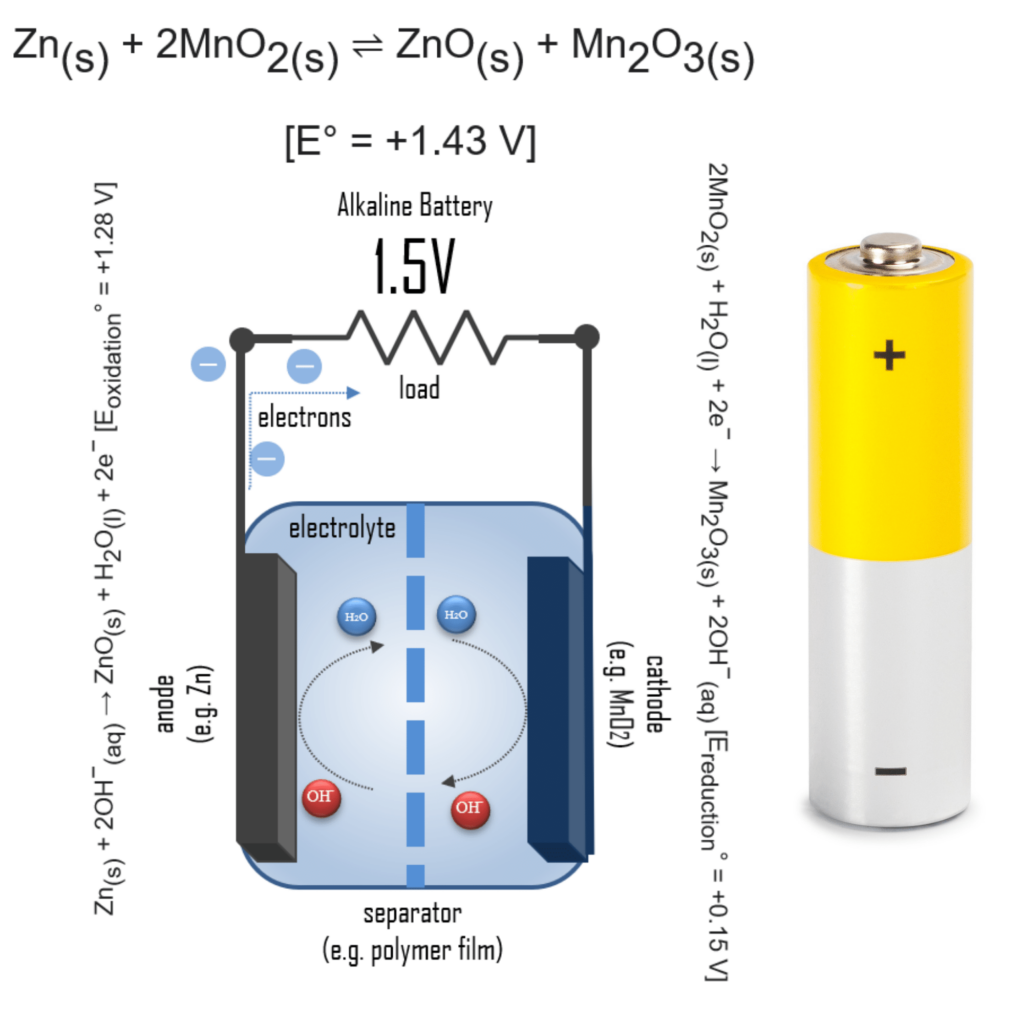
Why are alkaline batteries 1.5V?
In an alkaline battery, the negative electrode is zinc and the positive electrode is high-density manganese dioxide (MnO2). The alkaline electrolyte of potassium hydroxide, KOH, is not consumed during the reaction, only the zinc and MnO2 are consumed during discharge. The alkaline electrolyte of potassium hydroxide remains, as there are equal amounts of OH− consumed and produced.
The half-reactions are:
Zn(s) + 2OH−(aq) → ZnO(s) + H2O(l) + 2e− [Eoxidation° = +1.28 V]
2MnO2(s) + H2O(l) + 2e− → Mn2O3(s) + 2OH−(aq) [Ereduction° = +0.15 V]
Overall reaction:
Zn(s) + 2MnO2(s) ⇌ ZnO(s) + Mn2O3(s) [e° = +1.43 V]
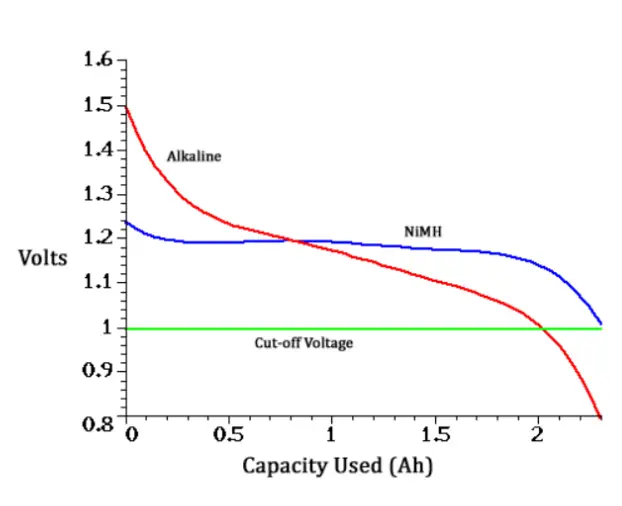
Why are alkaline batteries (AAA or AA) made to be 1.5V while rechargeables are 1.2V?
In general, batteries convert stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits.
- Primary (single-use or alkaline) batteries use cells that have 1.5V open circuit voltage when fresh.
- Secondary (rechargeable) batteries use cells from NiMh or NiCd, which have 1.2V open circuit voltage.
In practice, the alkaline batteries and the rechargeable batteries can be used interchangeably in sets. They have only different voltage characteristics. It is given by their different chemistry. Primary cells gradually drop in voltage from use; they start at 1.5 volts, drop to 1.2 and continue to 1.0 where the appliance stops working. Secondary cells operate more uniformly even with only 1.2 volts; they have flat discharge where they pretty much stay at 1.2 volts until depleted and then drop off very quickly to below 1.0 volts.
Since electronic devices are usually made to run from cell voltages of 1.0 to 1.5 volts, the alkaline batteries and the rechargeable batteries perform similarly. In fact, it’s generally considered that secondary 1.2 V cells work better than alkalines having lower output impedance and more consistent voltage from start to finish of a charge.
Other Characteristics
To compare and understand the capability of each battery, some important parameters are characteristic of each battery, also within a type of battery. These parameters are a reference when a battery is needed, and specific qualities are required since batteries are used in all types of devices and for infinite purposes.
Cell Voltage
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
Cut-off Voltage
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
Capacity
The coulometric capacity is the total Amp-hours available when the battery is discharged at a certain discharge current from 100% SOC to the cut-off voltage.
C-rate of Battery
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
Self-discharge
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied.
Degradation
Some degradation of rechargeable batteries occurs on each charge–discharge cycle. Degradation usually occurs because electrolyte migrates away from the electrodes or because active material detaches from the electrodes.
Depth of Discharge
Depth of discharge is a measure of how much energy has been withdrawn from a battery and is expressed as a percentage of full capacity. For example, a 100 Ah battery from which 40 Ah has been withdrawn has undergone a 40% depth of discharge (DOD).
State of Charge
The state of charge refers to the amount of charge in a battery relative to its predefined “full” and “empty” states i.e. the amount of charge in Amp-hours left in the battery.









