30-second summary
Self-discharge of Batteries
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied. The rate of side reactions is reduced for batteries stored at lower temperatures, although some can be damaged by freezing.
An electric battery is essentially a source of DC electrical energy. It converts stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. A typical battery consists of one or more voltaic cells.
The fundamental principle in an electrochemical cell is spontaneous redox reactions in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated.
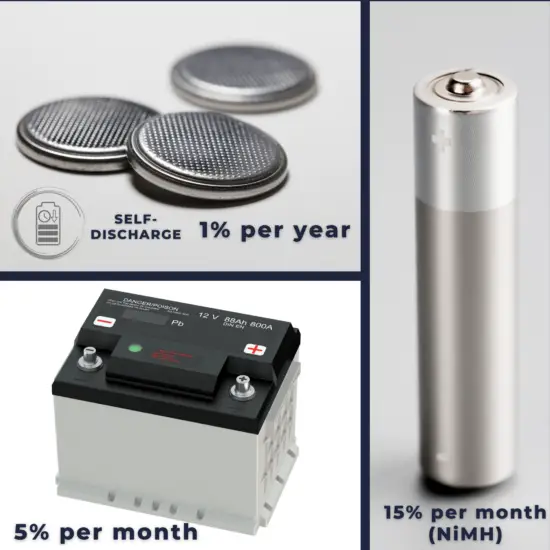
Self-discharge of Batteries
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied. The rate of side reactions is reduced for batteries stored at lower temperatures, although some can be damaged by freezing.
- Old rechargeable batteries self-discharge more rapidly than disposable alkaline batteries, especially nickel-based batteries; a freshly charged nickel-cadmium (NiCd) battery loses 10% of its charge in the first 24 hours and thereafter discharges at a rate of about 10% a month.
- The self-discharge of NIMH battery is 5–20% on the first day and stabilizes around 0.5–4% per day at room temperature. But at 45 °C it is approximately three times as high.
- A lead acid battery left in storage at moderate temperatures has an estimated self-discharge rate of 5% per month. This rate increases as temperatures rise and the risk of sulfation increases.
- Li-ion rechargeable batteries have a self-discharge rate typically stated by manufacturers to be 1.5–2% per month. The rate increases with temperature and state of charge.
- Primary batteries typically lose 2 to 5 percent of their original charge per year when stored at room temperature (20–30 °C).
- For the lithium-metal primary battery, such as the CR2032, the self-discharge rate is around 1% a year; this means a battery that is in storage.
A lower temperature reduces the rate of self-discharge, but manufacturers usually do not recommend such long-term storage.
Other Characteristics
To compare and understand the capability of each battery, some important parameters are characteristic of each battery, also within a type of battery. These parameters are a reference when a battery is needed, and specific qualities are required since batteries are used in all types of devices and for infinite purposes.
Cell Voltage
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
Cut-off Voltage
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
Capacity
The coulometric capacity is the total Amp-hours available when the battery is discharged at a certain discharge current from 100% SOC to the cut-off voltage.
C-rate of Battery
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
Self-discharge
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied.
Degradation
Some degradation of rechargeable batteries occurs on each charge–discharge cycle. Degradation usually occurs because electrolyte migrates away from the electrodes or because active material detaches from the electrodes.
Depth of Discharge
Depth of discharge is a measure of how much energy has been withdrawn from a battery and is expressed as a percentage of full capacity. For example, a 100 Ah battery from which 40 Ah has been withdrawn has undergone a 40% depth of discharge (DOD).
State of Charge
The state of charge refers to the amount of charge in a battery relative to its predefined “full” and “empty” states i.e. the amount of charge in Amp-hours left in the battery.









