Flow Battery
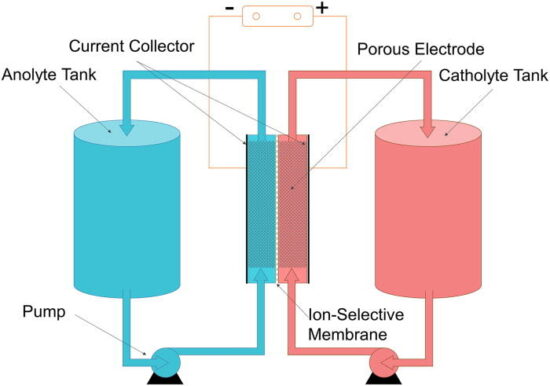
A flow battery, or redox flow battery, is a type of electrochemical cell that functions in much the same way as a traditional battery, except for the fact that the electrolyte solution is not stored within the cell but instead outside of the cell.
In the flow battery, chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane.
This has several benefits in terms of scalability, as well as in the operational lifetime of the battery. It also offers benefits in terms of the pricing structure of the batteries, as a much higher proportion of the investment capital required for these batteries is in the form of ongoing support and maintenance costs to maintain the requisite electrolyte solution levels rather than in the initial and ongoing hardware operational costs.
Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts. The energy capacity is a function of the electrolyte volume, and the power is a function of the surface area of the electrodes.
A flow battery may be used like a fuel cell (where the spent fuel is extracted and new fuel is added to the system) or like a rechargeable battery (where an electric power source drives regeneration of the fuel).
One of the most promising types of flow batteries, and the type included in this research, has been vanadium. Vanadium redox battery accumulates energy by exchanging (accepting/donating) electrons between electrolytes during the charging/discharging
process. They have really large cell voltage, which is beneficial to acquire large power
and energy than that of other redox flow batteries. Vanadium flow batteries offer somewhat greater simplicity because of their use of only one electrolyte solution, rather than two as is the case with most other flow batteries. They can be discharged completely without damaging the system components, and their stated cycle life is greater than 10,000 cycles. Their
overall cycle eciency, however, ranges from 65% to 72%, depending on the manufacturer (70% is the value used in the model). Vanadium flow batteries have a low energy-to-volume ratio, though this is less of a concern with grid-level energy storage applications.
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery