30-second summary
Zinc-air Battery
Zinc-air batteries are non-rechargeable and also mechanically rechargeable metal-air batteries powered by oxidizing zinc with oxygen from the air.
These batteries have high energy densities and are relatively inexpensive to produce. Zinc-air batteries offer specific and volumetric energy densities of around 500 Wh.kg−1 and 1000 Wh.L−1, respectively, which are among the highest for a battery system.
The commercially available primary zinc-air battery has a sticker attached over the air access holes, which is removed just before use. Once the sticker has been removed, it is recommended that the battery be used continuously.
Overall reaction:
Zn(s) +O2(g) ⇌ 2ZnO(s) [e° = +1.59 V]

An electric battery is essentially a source of DC electrical energy. It converts stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. A typical battery consists of one or more voltaic cells.
The fundamental principle in an electrochemical cell is spontaneous redox reactions in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated.
Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals. Even though a wide range of types of batteries exists with different combinations of materials, all of them use the same principle of the oxidation-reduction reaction. Batteries are designed so that the energetically favorable redox reaction can occur only when electrons move through the external part of the circuit.
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction. Because most of the resulting voltages are around 2V, cells are connected in series to obtain more practical electrical potentials (i.e. six 2V lead acid cells are connected in series to obtain a typical 12V battery).
Zinc-air Battery
Zinc-air batteries are non-rechargeable and also mechanically rechargeable metal-air batteries powered by oxidizing zinc with oxygen from the air. The zinc metal electrode forms the largest part of the cell and is the negative electrode. A solution of KOH or caustic soda works as an electrolyte and improves the standard potential. The separator isolates both electrodes mechanically in order to prevent electric conduction inside the cell. Oxygen from the air reacts at the cathode and forms hydroxyl ions which migrate into the zinc paste and form zincate, releasing electrons to travel to the cathode.
These batteries have high energy densities and are relatively inexpensive to produce. Zinc-air batteries offer specific and volumetric energy densities of around 500 Wh.kg−1 and 1000 Wh.L−1, respectively, which are among the highest for a battery system. The discharge profile is largely flat with a voltage of around 1.3 V. Size ranges from very small button cells for hearing aids, larger batteries used in film cameras that previously used mercury batteries, to very large batteries used for electric vehicle propulsion and grid-scale energy storage.
The major disadvantage is their limited power output, which is mainly due to the inadequate performance of air electrodes. The other major disadvantage is the dependence of both performance and operating period on ambient conditions such as humidity and temperature. The commercially available primary zinc-air battery has a sticker attached over the air access holes, which is removed just before use. Once the sticker has been removed, it is recommended that the battery be used continuously because the air access to the battery will inevitably result in water exchange and carbon dioxide contamination of the electrolyte, leading to the gradual deterioration of the battery. The storage characteristic of the zinc-air battery is satisfactory when the sticker is in place.
In general, metal-air batteries offer a high energy density because there is only one active mass inside the cell, and the cathodic reaction uses ambient air. Zinc is a very promising element because of its disposability, non-toxic behavior, and because it can be used as a secondary cell. Despite these advantages, this is a primary battery and cannot be recharged. For larger units, the batteries could be mechanically recharged by physically substituting the zinc electrode and electrolyte. Electrical recharge options are in development nowadays, but since this evolution is in its very prime stage, many years will be needed to achieve this technology.
Advantages and Disadvantages of Zinc-air Batteries
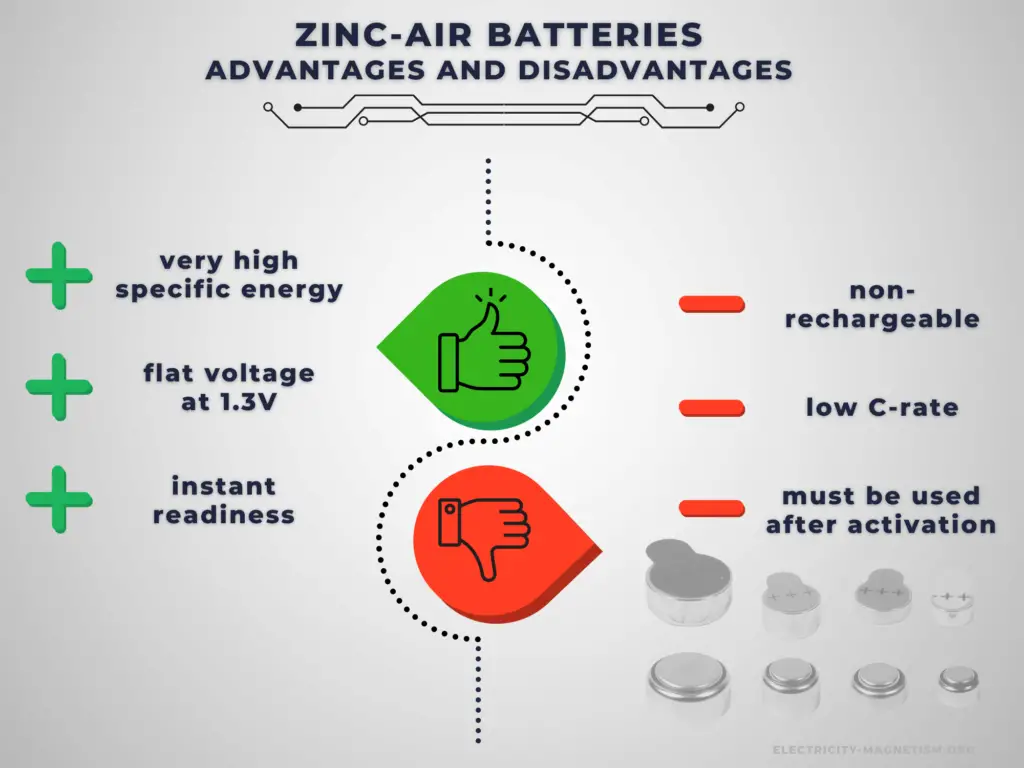
Advantages:
Primary batteries have higher energy density than rechargeable secondary cells. High specific energy, long storage times (low self-discharge), and instant readiness give primary batteries a unique advantage over other power sources. They are usually the best choice for low-drain applications. They can be carried to remote locations and used instantly, even after long storage; they are also readily available and environmentally friendly when disposed.
Zinc-air batteries offer specific and volumetric energy densities of around 500 Wh.kg−1 and 1000 Wh.L−1, respectively, which are among the highest for a battery system.
Disadvantages:
The main disadvantage of primary batteries is that they are non-rechargeable. Another disadvantage is their low C-rate. Even high current types are considered low in comparison to rechargeable batteries. They are also less environment friendly than rechargeable batteries. The application of primary batteries leads to a large amount of waste batteries to be recycled. For large batteries, primary batteries are usually not cost-effective.
The major disadvantage of zinc-air batteries is their limited power output, which is mainly due to the inadequate performance of air electrodes. The other major disadvantage is the dependence of both performance and operating period on ambient conditions such as humidity and temperature.
Characteristics of Zinc-air Batteries
To compare and understand the capability of each battery, some important parameters are characteristic of each battery, also within a type of battery. These parameters are a reference when a battery is needed, and specific qualities are required since batteries are used in all types of devices and for infinite purposes.
Cell Voltage
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
The nominal open circuit voltage for a zinc-air cell is 1.4 Volts. The operating voltage during discharge is dependent on the discharge load and the temperature. Typically, the operating voltage per cell is between 1.25 and 1.0 Volts.
Cut-off Voltage
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
The nominal cutoff voltage for zinc-air cells is 0.9 Volts. They can be discharged to lower values, but deep discharge to 0.5 Volts or less can result in electrolyte leakage from the air holes.
Capacity
The coulometric capacity is the total Amp-hours available when the battery is discharged at a certain discharge current from 100% SOC to the cut-off voltage.
Zinc-air batteries offer specific and volumetric energy densities of around 500 Wh.kg−1 and 1000 Wh.L−1, respectively, which are among the highest for a battery system.
C-rate of Battery
C-rate is used to express how fast a battery is discharged or charged relative to its maximum capacity. It has units h−1. A 1C rate means that the discharge current will discharge the entire battery in 1 hour.
Self-discharge
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied.
The zinc-air battery, when sealed, has an excellent shelf life, with a self-discharge rate of only 2 percent per year.
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery






