30-second summary
Nickel-cadmium Battery
The nickel-cadmium battery (Ni-Cd battery) is a type of secondary battery using nickel oxide hydroxide Ni(O)(OH) as a cathode and metallic cadmium as an anode.
The battery has low internal impedance resulting in high power capabilities but lower energy storage capacity compared to other battery systems. It has long cycle life and the capability of rapid recharge but may suffer from voltage depression or memory effect, meaning that the maximum charge voltage will decrease and hence the energy capacity if continuously discharged shallowly.
The overall reaction during discharge is:
2NiOOH + Cd + 2H2O → 2Ni(OH)2 + Cd(OH)2

Nickel-cadmium Battery
The nickel-cadmium battery (Ni-Cd battery) is a type of secondary battery using nickel oxide hydroxide Ni(O)(OH) as a cathode and metallic cadmium as an anode. The abbreviation Ni-Cd is derived from the chemical symbols of nickel (Ni) and cadmium (Cd).
The battery has low internal impedance resulting in high power capabilities but lower energy storage capacity compared to other battery systems. It has long cycle life and the capability of rapid recharge but may suffer from voltage depression or memory effect, meaning that the maximum charge voltage will decrease and hence the energy capacity if continuously discharged shallowly. The greatest disadvantage is the content of cadmium. Unfortunately, cadmium is extremely toxic; therefore, the Ni-Cd will not be an alternative for a modern battery system.
Nowadays, the applications of nickel-cadmium batteries are in small-size portable devices such as power tools, toys, emergency lighting, medical instrumentation, or industrial portable products. It is used in small-size products because their cost for low-power applications is inexpensive but three to four times more expensive than lead-acid batteries for the same capacity.
Chemistry of Nickel-cadmium Batteries – How it works
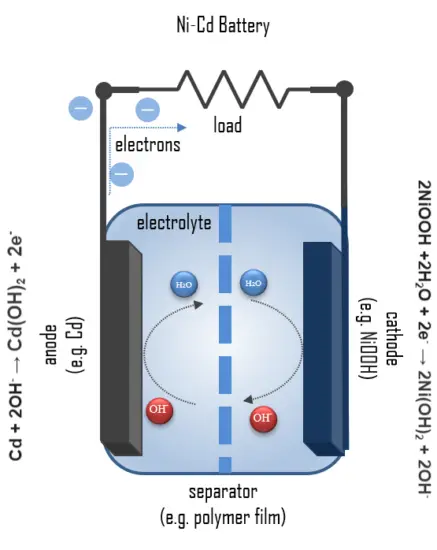
A fully charged Ni-Cd cell contains:
- Cathode: a nickel(III) oxide-hydroxide positive electrode plate
- Anode: a cadmium negative electrode plate
- Separator.
- Electrolyte: an alkaline electrolyte (potassium hydroxide).
Ni-Cd batteries usually have a metal case with a sealing plate equipped with a self-sealing safety valve. The positive and negative electrode plates, isolated from each other by the separator, are rolled in a spiral shape inside the case. This is known as the jelly-roll design and allows a Ni-Cd cell to deliver a much higher maximum current than an equivalent-size alkaline cell.
The positive electrode in the discharged state is composed of nickel hydroxide, which has been doped and modified to meet the battery requirements, and graphite as the conductive medium. The nickel cycles between two oxidation states during charge and discharge; upon the charge, the nickel hydroxide is converted into nickel oxyhydroxide (NiOOH):
2Ni(OH)2 + 2OH- →2NiOOH +2 H2O + 2e–
During discharge, the reactions at the nickel oxide electrode are:
2NiOOH +2H2O + 2e– → 2Ni(OH)2 + 2OH–
The negative electrode consists of cadmium hydroxide, Cd(OH)2, which is reduced to metallic cadmium during charging. The reaction is reversed throughout the discharge process, changing the oxidation state of cadmium from 0 to 2+, releasing two electrons per cadmium atom partaking in the reaction. Below is the reaction for the anode during charge:
Cd(OH)2 + 2e– → Cd + 2OH–
The chemical reactions at the cadmium electrode during discharge are:
Cd + 2OH– → Cd(OH)2 + 2e–
The overall reaction during discharge is:
2NiOOH + Cd + 2H2O → 2Ni(OH)2 + Cd(OH)2
Cadmium is a fairly hazardous metal on its own, therefore, a series of regulations and guidelines on how to handle it are in place by both governments all over the world and the EU.
The electrolyte acts as the ion charge carrier in batteries, and for Ni-Cd batteries, this mainly consists of concentrated potassium hydroxide, KOH, but may also have additions of sodium hydroxide, NaOH, and lithium hydroxide, LiOH.
Advantages and Disadvantages of Nickel-cadmium Batteries
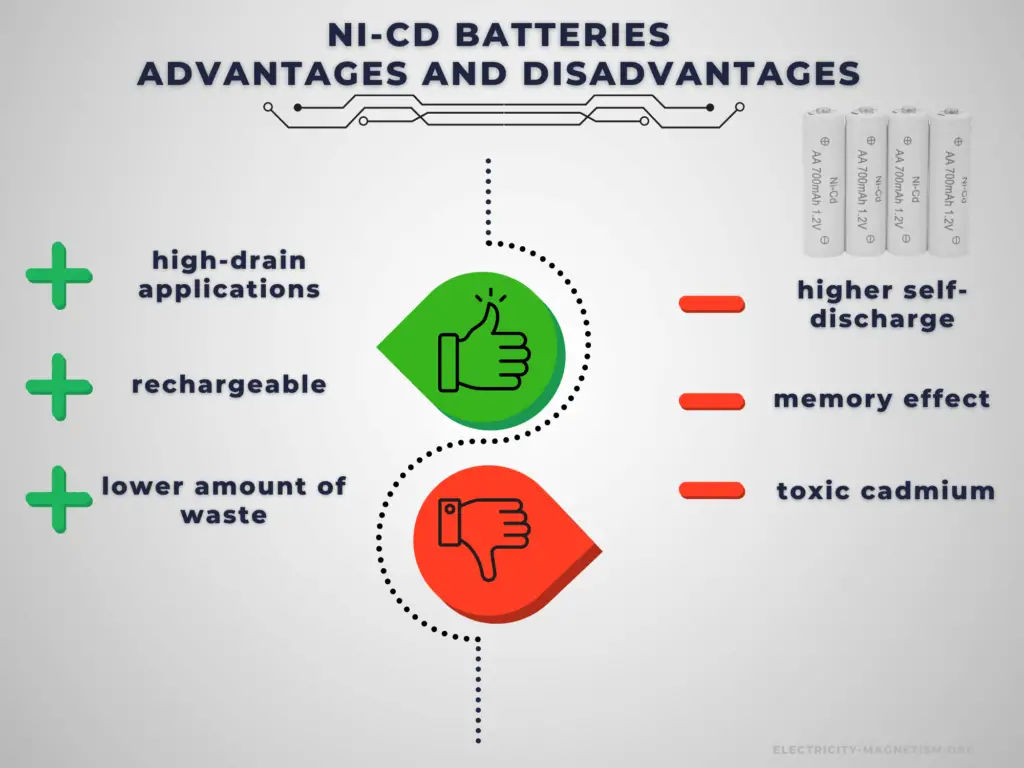
Advantages:
The main advantage of rechargeable cells is that they may be recharged after discharge. Therefore, rechargeable batteries are more environmentally friendly than primary batteries. Not only can they be used repeatedly, but they generate less waste over the long term. This is particularly true in the case of power-intensive devices, which consume batteries at an increased rate. Another very important advantage is a high C-rate. Rechargeable cells have better power output capabilities compared to primary cells and are used for high-power applications.
There are several specific advantages to Ni-Cd batteries.
- Delivers high current output.
- Relatively tolerant of overcharging.
- Withstands up to 500 charging cycles.
Disadvantages:
Battery price is one of the challenging factors in choosing the right rechargeable battery for your device or applications. It greatly affects the decision of the buyer. Rechargeable batteries have higher initial costs than their primary counterparts. Another important disadvantage is their self-discharge. In low-drain applications, the service life is more important, and the self-discharge characteristics of a rechargeable battery mean that they are less suitable for use as the primary energy source.
There are several specific disadvantages to Ni-Cd batteries.
- Cadmium is not environmentally friendly.
- Noticeable charging memory effect.
Characteristics of Nickel-cadmium Batteries
To compare and understand the capability of each battery, some important parameters are characteristic of each battery, also within a type of battery. These parameters are a reference when a battery is needed, and specific qualities are required since batteries are used in all types of devices and for infinite purposes.
Cell Voltage
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
A common open circuit voltage for Ni-Cd batteries (e.g. AAA and AA) is 1.2V.
Cut-off Voltage
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
When testing the capacity of a NiMH or NiCd battery a cut-off voltage of 1.0 V per cell is normally used, whereas 0.9 V is normally used as the cut-off voltage of an alkaline cell.
Capacity
The coulometric capacity is the total Amp-hours available when the battery is discharged at a certain discharge current from 100% SOC to the cut-off voltage.
Ni-Cd AA batteries feature a nominal voltage of 1.2 volts and an average capacity of 600-1000 mAh.
C-rate of Battery
C-rate is used to express how fast a battery is discharged or charged relative to its maximum capacity. It has units h−1. A 1C rate means that the discharge current will discharge the entire battery in 1 hour.
NiCd batteries designed for fast charging can be charged with currents that are several times the C-rating without extensive heat buildup.
Self-discharge
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied.
The self-discharge rate for a Ni-Cd battery is around 10%/month at 20 °C, and rising up to 20% at higher temperatures. It is recommended not to store Ni-Cd batteries for an extended amount of time without occasionally using the batteries.
Degradation
Some degradation of rechargeable batteries occurs on each charge-discharge cycle. Degradation usually occurs because electrolyte migrates away from the electrodes or because active material detaches from the electrodes.
Ni-Cd batteries can provide 300 to more than 500 discharge/charge cycles.
Depth of Discharge
Depth of discharge is a measure of how much energy has been withdrawn from a battery and is expressed as a percentage of full capacity. For example, a 100 Ah battery from which 40 Ah has been withdrawn has undergone a 40% depth of discharge (DOD).
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery
Frequently asked questions
There are two basic types of batteries: primary and secondary. Primary batteries are “single use” and cannot be recharged. Dry cells and (most) alkaline batteries are examples of primary batteries. The second type is rechargeable and is called a secondary battery.
Specialty Ni-Cd batteries are used in cordless and wireless telephones, emergency lighting, and other applications. With a relatively low internal resistance, they can supply high surge currents.
There are several specific advantages to Ni-Cd batteries. Delivers high current output. Relatively tolerant of overcharging. Withstands up to 500 charging cycles. On the other hand, there are several specific disadvantages to Ni-Cd batteries. Cadmium is not environmentally friendly. Noticeable charging memory effect.








