30-second summary
Secondary Battery
Secondary batteries, also known as secondary cells, or rechargeable batteries, are batteries that can be recharged by driving electric current in the opposite direction of the discharge current.
Primary cells have better energy storage capacity, but secondary cells have better power output capabilities compared to primary cells and are used for high-power applications. Rechargeable batteries are often more expensive, but in high-drain applications, they offer greater value as they can be reused. In low-drain applications, the service life is more important, and the self-discharge characteristics of a rechargeable battery mean that they are less suitable for use as the primary energy source.
Types of Secondary Batteries

An electric battery is essentially a source of DC electrical energy. It converts stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. A typical battery consists of one or more voltaic cells.
The fundamental principle in an electrochemical cell is spontaneous redox reactions in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated.
Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals. Even though a wide range of types of batteries exists with different combinations of materials, all of them use the same principle of the oxidation-reduction reaction. Batteries are designed so that the energetically favorable redox reaction can occur only when electrons move through the external part of the circuit.
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction. Because most of the resulting voltages are around 2V, cells are connected in series to obtain more practical electrical potentials (i.e. six 2V lead acid cells are connected in series to obtain a typical 12V battery).
Secondary Battery – Rechargeable Battery
Secondary batteries, also known as secondary cells, or rechargeable batteries, are batteries that can be recharged by driving electric current in the opposite direction of the discharge current. They must usually be charged before first use. Secondary batteries are (re)charged by applying electric current, which reverses the chemical reactions that occur during discharge/use. Devices to supply the appropriate current are called chargers. The oldest form of secondary battery is the lead–acid battery, which is widely used in automotive and boating applications.
Primary cells have better energy storage capacity, but secondary cells have better power output capabilities compared to primary cells and are used for high-power applications. Secondary batteries are often more expensive, but in high-drain applications, they offer greater value as they can be reused. In low-drain applications, the service life is more important, and the self-discharge characteristics of a rechargeable battery mean that they are less suitable for use as the primary energy source.
Types of Secondary Batteries
- Lithium-ion Battery. A lithium-ion battery, also known as a Li-ion battery, is a type of secondary (rechargeable) battery composed of cells in which lithium ions move from the anode through an electrolyte to the cathode during discharge and back when charging. Li-ion batteries, in general, have a high energy density, no memory effect, and low self-discharge. One of the most common types of cells is the 18650 battery, which is used in many laptop computer batteries, cordless power tools, certain electric cars, electric kick scooters, most e-bikes, portable power banks, and LED flashlights. The nominal voltage is 3.7 V.
- NiMH Battery. A nickel-metal hydride battery, NiMH, is a secondary battery with a positive electrode made of nickel hydroxide and a negative electrode made of a metal hydride (a hydrogen-absorbing alloy). The NiMH battery was commercially introduced in 1989 and was mainly used as a power source in portable personal computers. Since then, the NiMH battery system has become very popular in electric hybrid vehicles and makes up 10% of the total market for rechargeable batteries. Compared to the NiCd battery, the NiMH provides 40 percent higher specific energy resulting in about two times higher capacity. NiMH batteries are also less affected by the memory effect.
- Ni-Cd Battery. The nickel-cadmium battery (Ni-Cd battery) is a type of secondary battery using nickel oxide hydroxide Ni(O)(OH) as a cathode and metallic cadmium as an anode. The abbreviation Ni-Cd is derived from the chemical symbols of nickel (Ni) and cadmium (Cd). The battery has low internal impedance resulting in high power capabilities but lower energy storage capacity compared to other battery systems. It has long cycle life and capability of rapid recharge but may suffer from voltage depression or memory effect
- Lead-acid Battery. Lead-acid batteries contain liquid electrolyte in an unsealed container, requiring that the battery be kept upright and the area be well ventilated to ensure safe dispersal of the hydrogen gas it produces during overcharging. The lead–acid battery is relatively heavy for the amount of electrical energy it can supply. Its low manufacturing cost and its high surge current levels make it common where its capacity (over approximately 10 Ah) is more important than weight and handling issues. A common application is the modern car battery, which can, in general, deliver a peak current of 450 amperes.
Charging and discharging of Secondary Batteries – How it works
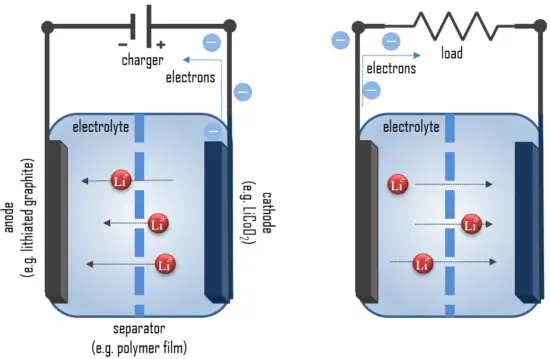
In simple terms, each battery is designed to keep the cathode and anode separated to prevent a reaction. The stored electrons will only flow when the circuit is closed. This happens when the battery is placed in a device and the device is turned on. Next, we will consider charging and discharging of lithium-ion batteries.
When the circuit is closed, the stronger attraction for the electrons by the cathode (e.g. LiCoO2 in lithium-ion batteries) will pull the electrons from the anode (e.g. lithium-graphite) through the wire in the circuit to the cathode electrode. This battery chemical reaction, this flow of electrons through the wire, is electricity.
If we go into detail, batteries convert chemical energy directly to electrical energy. Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals. Lithium metal is the lightest metal and possesses a high specific capacity (3.86 Ah/g) and an extremely low electrode potential (−3.04 V vs. standard hydrogen electrode). Therefore lithium is an ideal anode material for high-voltage and high-energy batteries.
During discharge, lithium is oxidized from Li to Li+ (0 to +1 oxidation state) in the lithium-graphite anode through the following reaction:
C6Li → 6C(graphite) + Li+ + e–
These lithium ions migrate through the electrolyte medium to the cathode, where they are incorporated into lithium cobalt oxide through the following reaction, which reduces cobalt from a +4 to a +3 oxidation state:
CoO2 (s) + Li+ + e– → LiCoO2 (s)
Here is the full reaction (left to right = discharging, right to left = charging):
C6Li + CoO2 ⇄ C6 + LiCoO2
These reactions can be run in reverse to recharge the cell. In this case, the lithium ions leave the lithium cobalt oxide cathode and migrate back to the anode, where they are reduced back to neutral lithium and reincorporated into the graphite network.
Batteries convert chemical energy directly to electrical energy. Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals.
Even though a wide range of types of batteries exists with different combinations of materials, all of them use the same principle of the oxidation-reduction reaction. In an electrochemical cell, spontaneous redox reactions take place in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated. The redox reaction is a chemical reaction that produces a change in the oxidation states of the atoms involved. Electrons are transferred from one element to another. As a result, the donor element, which is the anode, is oxidized (loses electrons), and the receiver element, the cathode, is reduced (gains electrons).
For example, the lithium-ion cell consists of two electrodes of dissimilar materials. The cathode is made of composite material and defines the name of the Li-ion battery cell. Cathode materials are generally constructed from LiCoO2 or LiMn2O4. Anode materials are traditionally constructed from graphite and other carbon materials. Graphite is the dominant material because of its low voltage and excellent performance. The electrolyte can be liquid, polymer (with a polymer gel as electrolyte) or solid. The separator is porous to enable the transport of lithium ions and prevents the cell from short-circuiting and thermal runaway.
During the charging process, Li+ ions move from the Li-containing anode and pass through the electrolyte-soaked separator and finally intercalating to the anode host structure. As a result, the electrons pass through the external circuit in the opposite direction.
During discharge, electrons flow from the negative electrode (anode) towards the positive electrode (cathode) through the external circuit. The reactions during discharge lower the chemical potential of the cell, so discharging transfers energy from the cell to wherever the electric current dissipates its energy, mostly in the external circuit. During charging, these reactions and transports go in the opposite direction.
Advantages and Disadvantages of Secondary Batteries
Advantages:
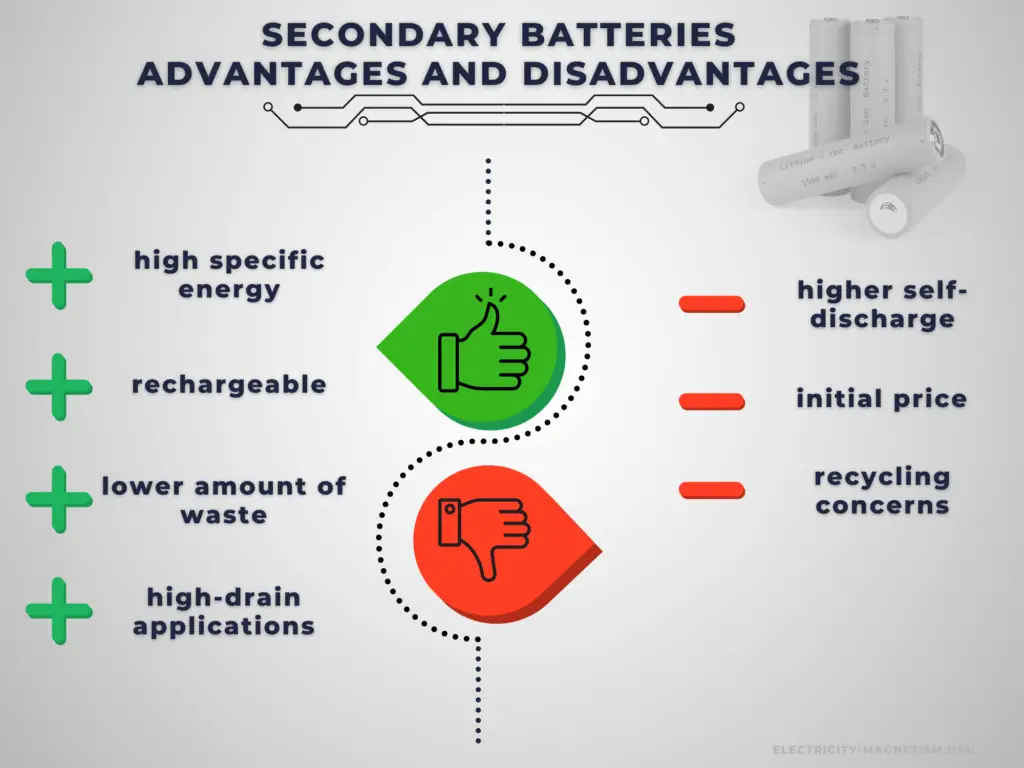
The main advantage of secondary cells is that they may be recharged after discharge. Therefore, rechargeable batteries are more environmentally friendly than primary batteries. Not only can they be used repeatedly, but they generate less waste over the long term. This is particularly true in the case of power-intensive devices which cunsume batteries at an increased rate. Another very important advantage is a high C-rate. Secondary cells have better power output capabilities compared to primary cells and are used for high-power applications.
Disadvantages:
Battery price is one of the challenging factors in choosing the right rechargeable battery for your device or applications. It greatly affects the decision of the buyer. Rechargeable batteries have higher initial costs than their primary counterparts. Another important disadvantage is their self-discharge. In low-drain applications, the service life is more important, and the self-discharge characteristics of a rechargeable battery mean that they are less suitable for use as the primary energy source.
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery
Frequently asked questions
There are two basic types of batteries: primary and secondary. Primary batteries are “single use” and cannot be recharged. Dry cells and (most) alkaline batteries are examples of primary batteries. The second type is rechargeable and is called a secondary battery.
In practice, alkaline batteries and rechargeable batteries can be used interchangeably in sets. They have only different voltage characteristics. It is given by their different chemistry. Primary cells gradually drop in voltage from use. They start at 1.5 volts, drop to 1.2 and continue to 1.0 where the appliance stops working. Secondary cells operate more uniformly, even with only 1.2 volts. They have flat discharge where they stay at 1.2 volts until depleted and drop off very quickly to below 1.0 volts.
The main advantage of secondary cells is that they may be recharged after discharge. Battery price is one of the challenging factors in choosing the right rechargeable battery for your device or applications.