30-second summary
12V Battery
The voltage of electric batteries is determined by:
- Chemistry. The potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
- Number of cells. Batteries in series produce a voltage equal to the number of batteries multiplied by the voltage of each individual battery.
The 12-volt battery is an electric battery that is typically composed of various cells. It is able to supply a nominal voltage of 12 volts.
There are various types of 12V batteries:
- Lead-acid 12V Battery. This battery is composed of 6 x 2V lead-acid cells. Lead-acid batteries are secondary (rechargeable) batteries that consist of a housing, two lead plates or groups of plates, one of them serving as a positive electrode and the other as a negative electrode, and a filling of 37% sulfuric acid (H2SO4) as electrolyte. The battery contains liquid electrolyte in an unsealed container, requiring it to be kept upright and the area well ventilated to ensure safe dispersal of the hydrogen gas it produces during overcharging. Lead acid batteries typically have coulombic efficiencies of 85% and energy efficiencies in the order of 70%.
- Alkaline 12V batteries. This battery is composed of 8 x 1.5V alkaline cells. For example, the A23 battery is a dry battery consisting of eight LR932 cells, with a nominal voltage of 12 V. It is mainly used in small electronic keychain radio devices, such as keyless vehicle entry systems, home security systems, garage door openers, and Bluetooth headsets.

An electric battery is essentially a source of DC electrical energy. It converts stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. A typical battery consists of one or more voltaic cells.
The voltage of electric batteries is determined by:
- Chemistry. The potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
- Number of cells. Batteries in series produce a voltage equal to the number of batteries multiplied by the voltage of each individual battery.
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
12V Battery
The 12-volt battery is an electric battery that is typically composed of various cells. It is able to supply a nominal voltage of 12 volts.
There are various types of 12V batteries:
- Lead-acid 12V Battery. This battery is composed of 6 x 2V lead-acid cells. Lead-acid batteries are secondary (rechargeable) batteries that consist of a housing, two lead plates or groups of plates, one of them serving as a positive electrode and the other as a negative electrode, and a filling of 37% sulfuric acid (H2SO4) as electrolyte. The battery contains liquid electrolyte in an unsealed container, requiring it to be kept upright and the area well ventilated to ensure safe dispersal of the hydrogen gas it produces during overcharging. Lead acid batteries typically have coulombic efficiencies of 85% and energy efficiencies in the order of 70%.
- Alkaline 12V batteries. This battery is composed of 8 x 1.5V alkaline cells. For example, the A23 battery is a dry battery consisting of eight LR932 cells, with a nominal voltage of 12 V. It is mainly used in small electronic keychain radio devices, such as keyless vehicle entry systems, home security systems, garage door openers, and Bluetooth headsets.
Lead-acid Battery
Lead-acid batteries are secondary (rechargeable) batteries that consist of a housing, two lead plates or groups of plates, one of them serving as a positive electrode and the other as a negative electrode, and a filling of 37% sulfuric acid (H2SO4) as electrolyte. The battery contains liquid electrolyte in an unsealed container, requiring it to be kept upright and the area well ventilated to ensure safe dispersal of the hydrogen gas it produces during overcharging. Lead acid batteries typically have coulombic efficiencies of 85% and energy efficiencies in the order of 70%.
Lead and lead dioxide, the active materials on the battery’s plates, react with sulfuric acid in the electrolyte to form lead sulfate. The lead sulfate first forms in a finely divided, amorphous state and easily reverts to lead, lead dioxide, and sulfuric acid when the battery recharges.
The lead–acid battery is relatively heavy for the amount of electrical energy it can supply. Its low manufacturing cost and its high surge current levels make it common where its capacity (over approximately 10 Ah) is more important than weight and handling issues. The disadvantage of this battery chemistry is that it is very sensitive to deep cycling compared to other battery systems, and due to the high density of lead, the specific energy of the batteries is quite low.
Most of the world’s lead–acid batteries are automobile starting, lighting, and ignition (SLI) batteries, with an estimated 320 million units shipped in 1999. In 1992 about 3 million tons of lead were used in the manufacture of batteries. Industrial fields of applications for lead acid batteries are as traction power for mining vehicles, forklifts and as stationary power sources such as emergency back up power storage (UPS) and signaling stations for railroads and telecommunication.
Alkaline Battery
An alkaline battery (IEC code: L) is a type of primary battery that provides direct electric current from the electrochemical reaction between zinc and manganese dioxide (MnO2) in the presence of an alkaline electrolyte.
The alkaline battery gets its name because it has an alkaline electrolyte of potassium hydroxide (KOH) instead of the acidic ammonium chloride (NH4Cl) or zinc chloride (ZnCl2) electrolyte of the zinc–carbon batteries. Other battery systems also use alkaline electrolytes, but they use different active materials for the electrodes.
The primary alkaline battery is a widely used product, which is essential for powering many portable devices, such as power tools, radios, toys, and remote controls. The most common size of alkaline battery is the well-known AA battery. Alkaline batteries are most commonly used in portable devices that have low current drains, are used only intermittently, or are used well away from an alternative power source, such as in alarm and communication circuits where other electric power is only intermittently available.
Cell Voltage

The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
The voltage produced by each lithium-ion cell is about 3.6 volts. This has many advantages. Being higher than that of the standard nickel-cadmium, nickel metal hydride, and even standard alkaline cells at around 1.5 volts and lead acid at around 2 volts per cell, the voltage of each lithium-ion cell is higher, requiring fewer cells in many battery applications.
Because most of the resulting voltages are around 2V, cells are connected in series to obtain more practical electrical potentials (i.e. 2V lead acid cells are connected in series to obtain a typical 12V battery).
Batteries with voltages greater than 1.5 volts are usually made up of cells connected in series inside a single case. In the 9 volt battery, there are six cells connected in series. The calculation is 6 × 1.5 Volt = 9 Volt.
To know the voltage of a battery, batteries are marked with nominal voltages which is the average voltage a cell outputs when is fully charged, but this may differ from the open circuit voltage.
In addition, some factors like low temperature can decrease the expected voltage output and increase with higher temperature, which is favorable for the electrochemical reactions.
To avoid batteries to discharge below a certain level which could cause damaging the battery, there is a voltage limit called cut-off voltage.
- 1.5V (DC) – A common open circuit voltage for non-rechargeable alkaline batteries (e.g. AAA, AA and C cells).
- 3V (DC) – Lithium-based primary cells are batteries that have metallic lithium as an anode. The voltage of most lithium-metal cells (e.g. button cells) is 3V.
- 3.8V (DC) – Almost all lithium-ion batteries work at 3.8 volts. In order to make current flow from the charger to the battery, there must be a potential difference. Therefore battery chargers or USBs for almost all smartphones provide a voltage of 5V.
- 12V (DC) – A common voltage for automobile batteries is 12 volts (DC). But this battery consists of six 2V lead cells.
Chemistry of Lead-acid Batteries – How it works
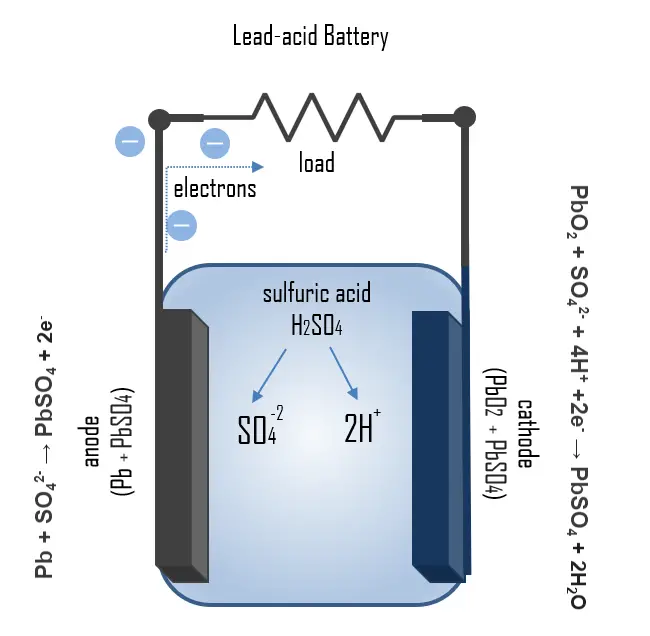
The principle of operation of the lead-acid battery can be illustrated by the chemical processes that take place during charging and discharging. During discharge, the process
Pb + SO42- → PbSO4 + 2e–
takes place at the anode. Lead is oxidized with the electrolyte to lead sulfate, releasing two electrons. Lead sulfate is also formed at the cathode by:
PbO2 + SO42- + 4H+ +2e– → PbSO4 + 2H2O
But in this reaction, a reduction of lead oxide takes place. Formed lead sulfate deposits as a coating on the electrodes and, to some extent also on the bottom of the housing. Since sulfuric acid is utilized during the discharge process, the SoC can be determined by measuring the density of the electrolyte.
During charging, the processes take place in the opposite direction so that the lead sulfate formed during discharging is oxidized to lead and reduced lead oxide, respectively. If the lead sulfate is completely consumed and the changing process is not stopped, electrolysis of the electrolyte begins. Overcharging with high charging voltages generates oxygen and hydrogen gas by electrolysis of water, which bubbles out and is lost. Sealed batteries have catalysts (Pd, Pt) above the vent where oxyhydrogen gas can recombine to water.
The resulting cell voltage can be determined from the galvanic series.
The total voltage of the redox reaction is thus:
E0 = 1.68V – ( – 0.36V) = 2.04V.
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery

