30-second summary
Primary Battery – Primary Cell
A primary battery or primary cell is a non-rechargeable battery that is designed to be used once discarded after use. This means that the redox reaction within the cell is not reversible like in a secondary (rechargeable) battery.
Primary cells have higher energy density than rechargeable secondary cells. High specific energy, long storage times (low self-discharge), and instant readiness give primary batteries a unique advantage over other power sources. They are usually the best choice for low-drain applications. They can be carried to remote locations and used instantly, even after long storage; they are also readily available and environmentally friendly when disposed.
The main disadvantage of primary batteries is that they are non-rechargeable. Another disadvantage is their low C-rate. Even high current types are considered low in comparison to rechargeable batteries. They are also less environment friendly than rechargeable batteries. The application of primary batteries leads to a large amount of waste batteries to be recycled. For large batteries, primary batteries are usually not cost-effective.

An electric battery is essentially a source of DC electrical energy. It converts stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. A typical battery consists of one or more voltaic cells.
The fundamental principle in an electrochemical cell is spontaneous redox reactions in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated.
Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals. Even though a wide range of types of batteries exists with different combinations of materials, all of them use the same principle of the oxidation-reduction reaction. Batteries are designed so that the energetically favorable redox reaction can occur only when electrons move through the external part of the circuit.
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction. Because most of the resulting voltages are around 2V, cells are connected in series to obtain more practical electrical potentials (i.e. six 2V lead acid cells are connected in series to obtain a typical 12V battery).
Primary Battery
A primary battery or primary cell is a non-rechargeable battery that is designed to be used once discarded after use. This means that the redox reaction within the cell is not reversible like in a secondary (rechargeable) battery. The primary alkaline battery is a widely used product, which is essential for powering many portable devices, such as power tools, radios, toys, and remote controls. These batteries are most commonly used in portable devices with low current drains, are used only intermittently, or are used well away from an alternative power source, such as in alarm and communication circuits where other electric power is only intermittently available.
Primary cells have higher energy density than the rechargeable secondary cell, but most types of primary cells have high inner impedance and will therefore cause a big voltage drop during high discharge current, limiting the power capacity. The most common primary batteries are based on zinc–manganese dioxide systems. There are three main types of primary batteries:
- Alkaline batteries (Zink/alkaline/Manganese Dioxide). An alkaline battery (IEC code: L) is a type of primary battery that provides direct electric current from the electrochemical reaction between zinc and manganese dioxide (MnO2) in the presence of an alkaline electrolyte. The alkaline battery gets its name because it has an alkaline electrolyte of potassium hydroxide (KOH) instead of the acidic ammonium chloride (NH4Cl) or zinc chloride (ZnCl2) electrolyte of the zinc–carbon batteries. Other battery systems also use alkaline electrolytes, but they use different active materials for the electrodes. The alkaline cell was introduced to the market in 1959 but did not become more common than the Zinc-carbon cell until around 1980.
- Zinc-carbon batteries. The zinc-carbon battery, also called the Leclanché cell, is a traditional general-purpose dry cell. Zinc–carbon batteries were the first commercial dry batteries developed from the technology of the wet Leclanché cell. This battery provides a direct electric current from the electrochemical reaction between zinc and manganese dioxide (MnO2) in the presence of an electrolyte. Zinc-carbon batteries today have been mostly replaced by more efficient and safe alkaline batteries. It produces a voltage of about 1.5 volts between the zinc anode, which is typically constructed as a cylindrical container for the battery cell, and a carbon rod surrounded by the cathode that collects the current from the manganese dioxide electrode. The name “zinc-carbon” is slightly misleading as it implies that carbon is acting as the reducing agent rather than the manganese dioxide.
- Zinc-chloride batteries. The zinc-chloride battery is a type of primary battery developed from a zinc-carbon cell. It is frequently referred to as a heavy-duty, extra-heavy-duty, super-heavy-duty, or super-extra-heavy-duty battery. Zinc-chloride cell is an improvement on the original zinc-carbon cell, using purer chemicals and giving a longer service life and steadier voltage output as it is used and offering about twice the service life of general-purpose zinc-carbon cells, or up to four times in continuous-use or high-drain applications.
The other common types of primary battery cells are:
- Silver-oxide battery. A silver-oxide battery is a primary cell using silver oxide as the cathode material and zinc for the anode. They are available in small sizes as button cells. The first letter in the IEC standard system identifies the chemical composition of the battery. S is for silver-oxide batteries (as SR516).
- Zinc-air battery. Zinc-air batteries are non-rechargeable and also mechanically rechargeable metal-air batteries powered by oxidizing zinc with oxygen from the air. These batteries have high energy densities and are relatively inexpensive to produce.
- Lithium-based primary cells are batteries that have metallic lithium as an anode. These types of batteries are also referred to as lithium-metal batteries. They have the lowest self-discharge rate hence the longest available shelf time, up to 10 years, and in temperatures up to 70. The first letter in the IEC standard system identifies the chemical composition of the battery. C is for silver-oxide batteries (as CR2032). Using an iron disulfide cathode gives a battery with a nominal voltage of 1.5 volts. This cell is used for high-performance AA batteries.
Advantages and Disadvantages of Primary Batteries
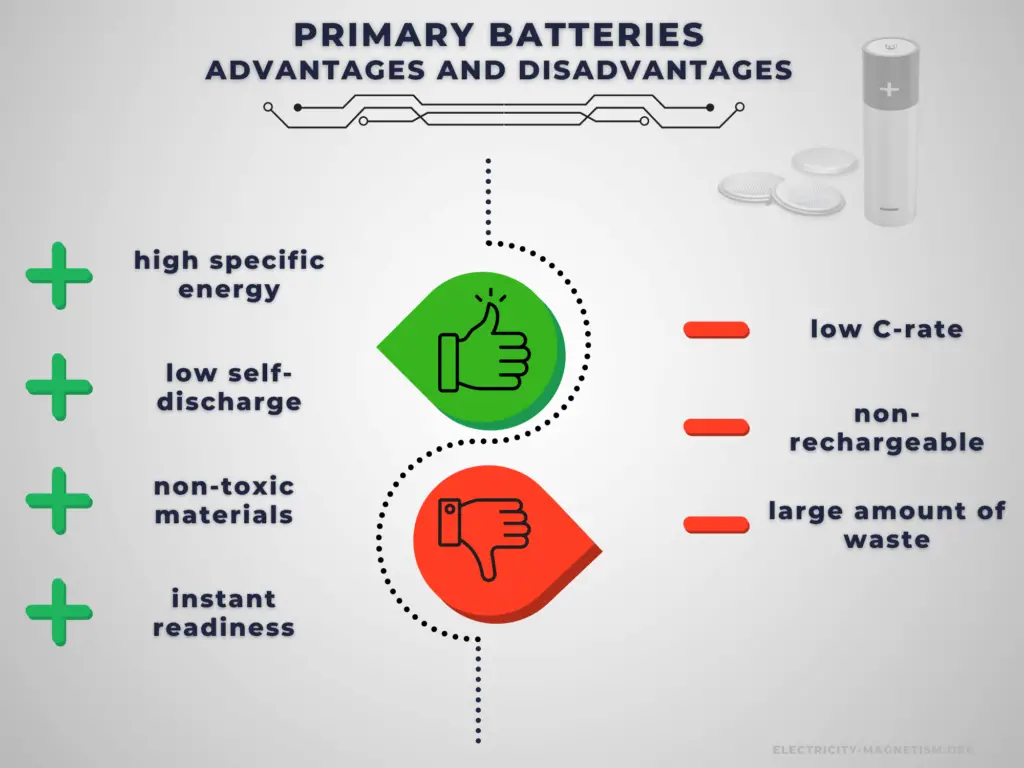
Advantages:
Primary cells have higher energy density than rechargeable secondary cells. High specific energy, long storage times (low self-discharge), and instant readiness give primary batteries a unique advantage over other power sources. They are usually the best choice for low-drain applications. They can be carried to remote locations and used instantly, even after long storage; they are also readily available and environmentally friendly when disposed.
Disadvantages:
The main disadvantage of primary batteries is that they are non-rechargeable. Another disadvantage is their low C-rate. Even high current types are considered low in comparison to rechargeable batteries. They are also less environment friendly than rechargeable batteries. The application of primary batteries leads to a large amount of waste batteries to be recycled. For large batteries, primary batteries are usually not cost-effective.
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery
Frequently asked questions
There are two basic types of batteries: primary and secondary. Primary batteries are “single use” and cannot be recharged. Dry cells and (most) alkaline batteries are examples of primary batteries. The second type is rechargeable and is called a secondary battery.
In practice, alkaline batteries and rechargeable batteries can be used interchangeably in sets. They have only different voltage characteristics. It is given by their different chemistry. Primary cells gradually drop in voltage from use. They start at 1.5 volts, drop to 1.2 and continue to 1.0 where the appliance stops working. Secondary cells operate more uniformly, even with only 1.2 volts. They have flat discharge where they stay at 1.2 volts until depleted and drop off very quickly to below 1.0 volts.
Primary cells have higher energy density and very low self-discharge. The main disadvantage of primary batteries is that they are non-rechargeable.

