30-second summary
AAA Battery
An AAA battery is one of the most common types of single cell cylindrical batteries. AAA batteries are common in portable electronic devices, toys, and remote controls.
Several different chemistries are used in their construction. The exact terminal voltage, capacity and practical discharge rates depend on cell chemistry; however, devices designed for AAA cells will usually only take 1.2-1.5 V unless specified by the manufacturer.
Types of AAA Batteries:

An electric battery is essentially a source of DC electrical energy. It converts stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. A typical battery consists of one or more voltaic cells.
The fundamental principle in an electrochemical cell is spontaneous redox reactions in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated.
An AAA battery is one of the most common types of single-cell cylindrical batteries. AAA batteries are common in portable electronic devices.
Dimensions and Weight of AAA Battery
An AAA battery is a single cell that measures 10.5 mm (0.41 in) in diameter and 44.5 mm (1.75 in) in length, including the positive terminal button, which is a minimum 0.8 mm (0.031 in).
The positive terminal has a maximum diameter of 3.8 mm (0.15 in); the flat negative terminal has a minimum diameter of 4.3 mm (0.17 in).
Alkaline AAA batteries weigh around 11.5 grams (0.41 oz), while primary lithium AAA batteries weigh about 7.6 g (0.27 oz). Rechargeable nickel–metal hydride (NiMH) AAA batteries are heavier, they typically weigh 14–15 g (0.49–0.53 oz).
Types of AAA Batteries
AAA batteries are basically divided into primary and secondary. Several different chemistries are used in their construction. The exact terminal voltage, capacity and practical discharge rates depend on cell chemistry; however, devices designed for AAA cells will usually only take 1.2-1.5 V unless specified by the manufacturer.
Primary AAA Battery. A primary battery or primary cell is a non-rechargeable battery that is designed to be used once discarded after use. These batteries are most commonly used in portable devices with low current drains, are used only intermittently, or are used well away from an alternative power source, such as in alarm and communication circuits where other electric power is only intermittently available. Primary cells have higher energy density than the rechargeable secondary cell, but most types of primary cells have high inner impedance and will therefore cause a big voltage drop during high discharge current, limiting the power capacity.
- Alkaline battery. An alkaline battery (IEC code: L) is a type of primary battery that provides direct electric current from the electrochemical reaction between zinc and manganese dioxide (MnO2) in the presence of an alkaline electrolyte. The alkaline battery gets its name because it has an alkaline electrolyte of potassium hydroxide (KOH) instead of the acidic ammonium chloride (NH4Cl) or zinc chloride (ZnCl2) electrolyte of the zinc–carbon batteries. The alkaline cell was introduced to the market in 1959 but did not become more common than the Zinc-carbon cell until around 1980.
- Lithium metal battery. Lithium-based primary cells are batteries that have metallic lithium as an anode. They have the lowest self-discharge rate hence the longest available shelf time, up to 10 years, and in temperatures up to 70. Using an iron disulfide cathode gives a battery with a nominal voltage of 1.5 volts. This cell is used for high-performance AA batteries.
- Zinc-carbon battery. The zinc-carbon battery, also called the Leclanché cell, is a traditional general-purpose dry cell. Zinc–carbon batteries were the first commercial dry batteries developed from the technology of the wet Leclanché cell. This battery provides a direct electric current from the electrochemical reaction between zinc and manganese dioxide (MnO2) in the presence of an electrolyte. It produces a voltage of about 1.5 volts between the zinc anode, which is typically constructed as a cylindrical container for the battery cell, and a carbon rod surrounded by the cathode that collects the current from the manganese dioxide electrode. The name “zinc-carbon” is slightly misleading as it implies that carbon is acting as the reducing agent rather than the manganese dioxide.
- Zinc-chloride battery. The zinc-chloride battery is a type of primary battery developed from a zinc-carbon cell. It is frequently referred to as a heavy-duty, extra-heavy-duty, super-heavy-duty, or super-extra-heavy-duty battery. Zinc-chloride cell is an improvement on the original zinc-carbon cell, using purer chemicals and giving a longer service life and steadier voltage output as it is used and offering about twice the service life of general-purpose zinc-carbon cells, or up to four times in continuous-use or high-drain applications.
Secondary AAA Battery. Secondary batteries, also known as secondary cells, or rechargeable batteries, are batteries that can be recharged by driving electric current in the opposite direction of the discharge current. Rechargeable batteries are often more expensive, but in high-drain applications, they offer greater value as they can be reused. In low-drain applications, the service life is more important, and the self-discharge characteristics of a rechargeable battery mean that they are less suitable for use as the primary energy source.
- NiMH battery. A nickel-metal hydride battery, NiMH, is a secondary battery with a positive electrode made of nickel hydroxide and a negative electrode made of a metal hydride (a hydrogen-absorbing alloy). The NiMH battery was commercially introduced in 1989 and was mainly used as a power source in portable personal computers. Since then, the NiMH battery system has become very popular in electric hybrid vehicles and makes up 10% of the total market for rechargeable batteries. Compared to the NiCd battery, the NiMH provides 40 percent higher specific energy resulting in about two times higher capacity. NiMH batteries are also less affected by the memory effect.
- NiCd battery. The nickel-cadmium battery (Ni-Cd battery) is a type of secondary battery using nickel oxide hydroxide Ni(O)(OH) as a cathode and metallic cadmium as an anode. The abbreviation Ni-Cd is derived from the chemical symbols of nickel (Ni) and cadmium (Cd). The battery has low internal impedance resulting in high power capabilities but lower energy storage capacity compared to other battery systems. It has long cycle life and capability of rapid recharge but may suffer from voltage depression or memory effect
Characteristics of AAA Batteries
To compare and understand the capability of each battery, some important parameters are characteristic of each battery, also within a type of battery. These parameters are a reference when a battery is needed, and specific qualities are required since batteries are used in all types of devices and for infinite purposes.
Cell Voltage
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
Alkaline batteries have an open cell voltage of about 1.5 V. A common open circuit voltage for NiMH batteries (e.g. AAA and AA) is 1.2V.
Cut-off Voltage
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
When testing the capacity of a NiMH or NiCd battery, a cut-off voltage of 1.0 V per cell is normally used, whereas 0.9 V is normally used as the cut-off voltage of an alkaline cell.
Capacity
The coulometric capacity is the total Amp-hours available when the battery is discharged at a certain discharge current from 100% SOC to the cut-off voltage.
A typical alkaline or NiMH battery in the standard “AAA” size has about 800 (NiMH) to 1100 mAh (alkaline).
C-rate of Battery
C-rate is used to express how fast a battery is discharged or charged relative to its maximum capacity. It has units h−1. A 1C rate means that the discharge current will discharge the entire battery in 1 hour.
To obtain a reasonably good capacity reading, manufacturers commonly rate alkaline and lead acid batteries at a very low 0.05C, or a 20-hour discharge.
Self-discharge
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied.
One of the main advantages of alkaline batteries is that they are easy to store. They are chemically stable, and they have a very low self-discharge rate. Alkaline batteries typically lose 2 to 3 percent of their original charge per year when stored at room temperature (20–30 °C). The NiMH battery also has high self-discharge and can lose up to 20 % of its charge during the first 24 hours and thereafter 10 % per month.
Degradation
Some degradation of rechargeable batteries occurs on each charge-discharge cycle. Degradation usually occurs because electrolyte migrates away from the electrodes or because active material detaches from the electrodes.
The cycle life for NiMH batteries is typically 700-1,000 life cycles.
Chemistry of Alkaline Batteries
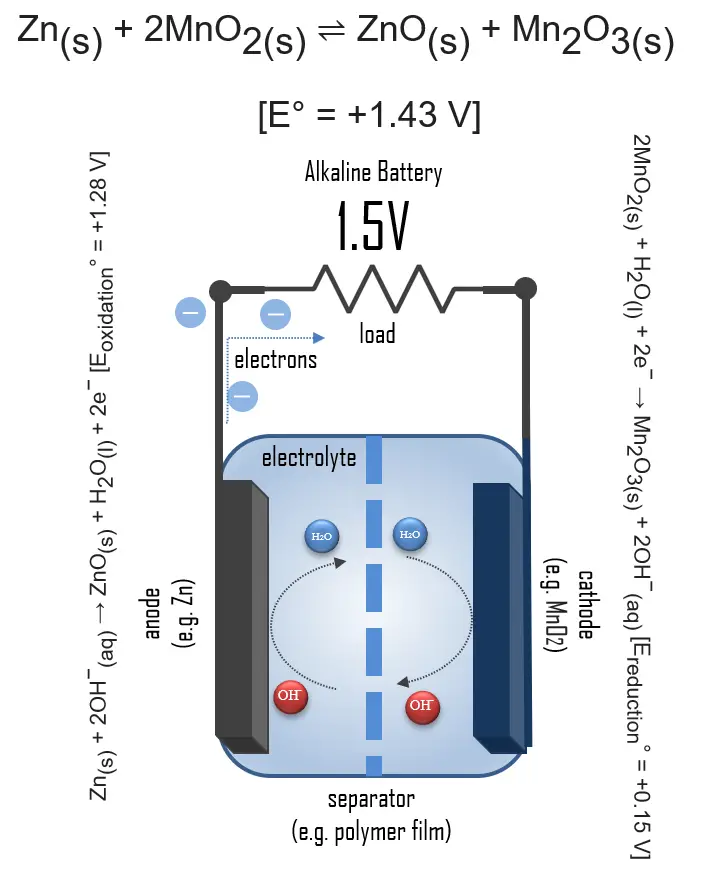
In simple terms, each battery is designed to keep the cathode and anode separated to prevent a reaction. The stored electrons will only flow when the circuit is closed. This happens when the battery is placed in a device, and the device is turned on.
When the circuit is closed, the stronger attraction for the electrons by the cathode (e.g. manganese dioxide in alkaline batteries) will pull the electrons from the anode (e.g. zinc) through the wire in the circuit to the cathode electrode. This battery chemical reaction, this flow of electrons through the wire, is electricity.
If we go into detail, batteries convert chemical energy directly to electrical energy. Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals.
Even though a wide range of types of batteries exists with different combinations of materials, all of them use the same principle of the oxidation-reduction reaction. In an electrochemical cell, spontaneous redox reactions take place in two electrodes separated by an electrolyte, which is an ionic conductive and electrically insulated substance. The redox reaction is a chemical reaction that produces a change in the oxidation states of the atoms involved. Electrons are transferred from one element to another. As a result, the donor element, which is the anode, is oxidized (loses electrons), and the receiver element, the cathode, is reduced (gains electrons).
In an alkaline battery, the negative electrode is zinc, and the positive electrode is high-density manganese dioxide (MnO2). The alkaline electrolyte of potassium hydroxide, KOH, is not consumed during the reaction. Only the zinc and MnO2 are consumed during discharge. The alkaline electrolyte of potassium hydroxide remains, as there are equal amounts of OH− consumed and produced.
The half-reactions are:
Zn(s) + 2OH−(aq) → ZnO(s) + H2O(l) + 2e− [Eoxidation° = +1.28 V]
2MnO2(s) + H2O(l) + 2e− → Mn2O3(s) + 2OH−(aq) [Ereduction° = +0.15 V]
Overall reaction:
Zn(s) + 2MnO2(s) ⇌ ZnO(s) + Mn2O3(s) [e° = +1.43 V]
Applying this battery chemistry to the real world, the electrons generated during the reaction are used to power devices when the circuit is closed.
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery







