30-second summary
9V Battery
The voltage of electric batteries is determined by:
- Chemistry. The potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
- Number of cells. Batteries in series produce a voltage equal to the number of batteries multiplied by the voltage of each individual battery.
The nine-volt battery, or 9-volt battery, is an electric battery that is typically composed of 6 x 1.5V alkaline cells. Therefore, it supplies a nominal voltage of 9 volts.
9V batteries are usually primary batteries:
- Alkaline batteries
- Zinc-carbon batteries
- NiMH batteries
Overall reaction:
Zn(s) + 2MnO2(s) ⇌ ZnO(s) + Mn2O3(s) [e° = +1.43 V]

An electric battery is essentially a source of DC electrical energy. It converts stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. A typical battery consists of one or more voltaic cells.
The voltage of electric batteries is determined by:
- Chemistry. The potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
- Number of cells. Batteries in series produce a voltage equal to the number of batteries multiplied by the voltage of each individual battery.
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
9V Battery
The nine-volt battery, or 9-volt battery, is an electric battery that is typically composed of 6 x 1.5V alkaline cells. Therefore, it supplies a nominal voltage of 9 volts. Actual voltage measures 7.2 to 9.6 volts, depending on battery chemistry. Batteries of various sizes and capacities are manufactured; a very common size is known as PP3 (Power Pack – 3). The PP3 has a rectangular prism shape with rounded edges and two polarized snap connectors on the top. This type is commonly used for many applications including household uses such as smoke and gas detectors, clocks, and toys.
The nine-volt PP3-size battery is commonly available in primary zinc-carbon and alkaline chemistry, in primary lithium iron disulfide and lithium manganese dioxide (sometimes designated CRV9), and in rechargeable form in nickel-cadmium (Ni–Cd), nickel-metal hydride (Ni–MH) and lithium-ion.
There are three main types of nine-volt PP3-size batteries:
- Alkaline batteries (Zink/alkaline/Manganese Dioxide). An alkaline battery (IEC code: L) is a type of primary battery that provides direct electric current from the electrochemical reaction between zinc and manganese dioxide (MnO2) in the presence of an alkaline electrolyte. The alkaline battery gets its name because it has an alkaline electrolyte of potassium hydroxide (KOH) instead of the acidic ammonium chloride (NH4Cl) or zinc chloride (ZnCl2) electrolyte of the zinc–carbon batteries. Other battery systems also use alkaline electrolytes, but they use different active materials for the electrodes. The alkaline cell was introduced to the market in 1959 but did not become more common than the Zinc-carbon cell until around 1980.
- Zinc-carbon batteries. The zinc-carbon battery, also called the Leclanché cell, is a traditional general-purpose dry cell. Zinc–carbon batteries were the first commercial dry batteries developed from the technology of the wet Leclanché cell. This battery provides a direct electric current from the electrochemical reaction between zinc and manganese dioxide (MnO2) in the presence of an electrolyte. Zinc-carbon batteries today have been mostly replaced by more efficient and safe alkaline batteries. It produces a voltage of about 1.5 volts between the zinc anode, which is typically constructed as a cylindrical container for the battery cell, and a carbon rod surrounded by the cathode that collects the current from the manganese dioxide electrode. The name “zinc-carbon” is slightly misleading as it implies that carbon is acting as the reducing agent rather than the manganese dioxide.
- NiMH batteries. A nickel metal hydride battery, NiMH, is a rechargeable battery with a positive electrode made of nickel hydroxide and a negative electrode made of a metal hydride (a hydrogen-absorbing alloy). The NiMH battery was commercially introduced in 1989 and was mainly used as a power source in portable personal computers. Since then, the NiMH battery system has become very popular in electric hybrid vehicles and makes up 10% of the total market for rechargeable batteries. Compared to the NiCd battery, the NiMH provides 40 percent higher specific energy resulting in about two times higher capacity. NiMH batteries are also less affected by voltage depression, but the main advantage is the absence of toxic cadmium. The memory effect of NiMH batteries is much less than nickel-cadmium batteries.
Cell Voltage

The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
The voltage produced by each lithium-ion cell is about 3.6 volts. This has many advantages. Being higher than that of the standard nickel-cadmium, nickel metal hydride, and even standard alkaline cells at around 1.5 volts and lead acid at around 2 volts per cell, the voltage of each lithium-ion cell is higher, requiring fewer cells in many battery applications.
Because most of the resulting voltages are around 2V, cells are connected in series to obtain more practical electrical potentials (i.e. 2V lead acid cells are connected in series to obtain a typical 12V battery).
Batteries with voltages greater than 1.5 volts are usually made up of cells connected in series inside a single case. In the 9 volt battery, there are six cells connected in series. The calculation is 6 × 1.5 Volt = 9 Volt.
To know the voltage of a battery, batteries are marked with nominal voltages which is the average voltage a cell outputs when is fully charged, but this may differ from the open circuit voltage.
In addition, some factors like low temperature can decrease the expected voltage output and increase with higher temperature, which is favorable for the electrochemical reactions.
To avoid batteries to discharge below a certain level which could cause damaging the battery, there is a voltage limit called cut-off voltage.
- 1.5V (DC) – A common open circuit voltage for non-rechargeable alkaline batteries (e.g. AAA, AA and C cells).
- 3V (DC) – Lithium-based primary cells are batteries that have metallic lithium as an anode. The voltage of most lithium-metal cells (e.g. button cells) is 3V.
- 3.8V (DC) – Almost all lithium-ion batteries work at 3.8 volts. In order to make current flow from the charger to the battery, there must be a potential difference. Therefore battery chargers or USBs for almost all smartphones provide a voltage of 5V.
- 12V (DC) – A common voltage for automobile batteries is 12 volts (DC). But this battery consists of six 2V lead cells.
Chemistry of Alkaline Batteries
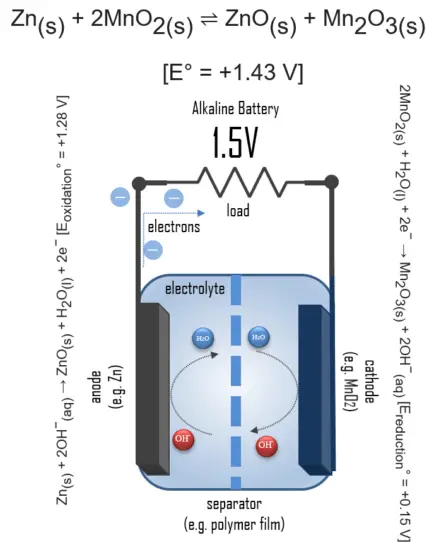
In simple terms, each battery is designed to keep the cathode and anode separated to prevent a reaction. The stored electrons will only flow when the circuit is closed. This happens when the battery is placed in a device, and the device is turned on.
When the circuit is closed, the stronger attraction for the electrons by the cathode (e.g. manganese dioxide in alkaline batteries) will pull the electrons from the anode (e.g. zinc) through the wire in the circuit to the cathode electrode. This battery chemical reaction, this flow of electrons through the wire, is electricity.
If we go into detail, batteries convert chemical energy directly to electrical energy. Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals.
Even though a wide range of types of batteries exists with different combinations of materials, all of them use the same principle of the oxidation-reduction reaction. In an electrochemical cell, spontaneous redox reactions take place in two electrodes separated by an electrolyte, which is an ionic conductive and electrically insulated substance. The redox reaction is a chemical reaction that produces a change in the oxidation states of the atoms involved. Electrons are transferred from one element to another. As a result, the donor element, which is the anode, is oxidized (loses electrons), and the receiver element, the cathode, is reduced (gains electrons).
In an alkaline battery, the negative electrode is zinc, and the positive electrode is high-density manganese dioxide (MnO2). The alkaline electrolyte of potassium hydroxide, KOH, is not consumed during the reaction. Only the zinc and MnO2 are consumed during discharge. The alkaline electrolyte of potassium hydroxide remains, as there are equal amounts of OH− consumed and produced.
The half-reactions are:
Zn(s) + 2OH−(aq) → ZnO(s) + H2O(l) + 2e− [Eoxidation° = +1.28 V]
2MnO2(s) + H2O(l) + 2e− → Mn2O3(s) + 2OH−(aq) [Ereduction° = +0.15 V]
Overall reaction:
Zn(s) + 2MnO2(s) ⇌ ZnO(s) + Mn2O3(s) [e° = +1.43 V]
Applying this battery chemistry to the real world, the electrons generated during the reaction are used to power devices when the circuit is closed.
Why are alkaline batteries (AAA or AA) made to be 1.5V while rechargeables are 1.2V?
In general, batteries convert stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits.
- Primary (single-use or alkaline) batteries use cells that have 1.5V open circuit voltage when fresh.
- Secondary (rechargeable) batteries use cells from NiMh or NiCd, which have 1.2V open circuit voltage.
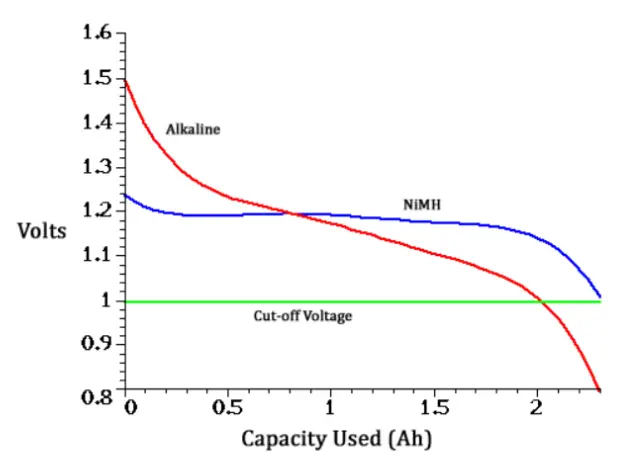
In practice, alkaline batteries and rechargeable batteries can be used interchangeably in sets. They have only different voltage characteristics. It is given by their different chemistry. Primary cells gradually drop in voltage from use. They start at 1.5 volts, drop to 1.2 and continue to 1.0 where the appliance stops working. Secondary cells operate more uniformly, even with only 1.2 volts. They have flat discharge where they pretty much stay at 1.2 volts until depleted and then drop off very quickly to below 1.0 volts.
Since electronic devices are usually made to run from cell voltages of 1.0 to 1.5 volts, alkaline batteries and rechargeable batteries perform similarly. In fact, it’s generally considered that secondary 1.2 V cells work better than alkalines having lower output impedance and more consistent voltage from start to finish of a charge.
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery

