30-second summary
Lead-acid Battery
Lead-acid batteries are secondary (rechargeable) batteries that consist of a housing, two lead plates or groups of plates, one of them serving as a positive electrode and the other as a negative electrode, and a filling of 37% sulfuric acid (H2SO4) as electrolyte.
Most of the world’s lead–acid batteries are automobile starting, lighting, and ignition (SLI) batteries, with an estimated 320 million units shipped in 1999. In 1992 about 3 million tons of lead were used in the manufacture of batteries. Industrial fields of applications for lead acid batteries are as traction power for mining vehicles, forklifts and as stationary power sources such as emergency back up power storage (UPS) and signaling stations for railroads and telecommunication.

Lead-acid Battery
Lead-acid batteries are secondary (rechargeable) batteries that consist of a housing, two lead plates or groups of plates, one of them serving as a positive electrode and the other as a negative electrode, and a filling of 37% sulfuric acid (H2SO4) as electrolyte. The battery contains liquid electrolyte in an unsealed container, requiring it to be kept upright and the area well ventilated to ensure safe dispersal of the hydrogen gas it produces during overcharging. Lead acid batteries typically have coulombic efficiencies of 85% and energy efficiencies in the order of 70%.
Lead and lead dioxide, the active materials on the battery’s plates, react with sulfuric acid in the electrolyte to form lead sulfate. The lead sulfate first forms in a finely divided, amorphous state and easily reverts to lead, lead dioxide, and sulfuric acid when the battery recharges.
The lead–acid battery is relatively heavy for the amount of electrical energy it can supply. Its low manufacturing cost and its high surge current levels make it common where its capacity (over approximately 10 Ah) is more important than weight and handling issues. The disadvantage of this battery chemistry is that it is very sensitive to deep cycling compared to other battery systems, and due to the high density of lead, the specific energy of the batteries is quite low.
Most of the world’s lead–acid batteries are automobile starting, lighting, and ignition (SLI) batteries, with an estimated 320 million units shipped in 1999. In 1992 about 3 million tons of lead were used in the manufacture of batteries. Industrial fields of applications for lead acid batteries are as traction power for mining vehicles, forklifts and as stationary power sources such as emergency back up power storage (UPS) and signaling stations for railroads and telecommunication.
Composition of Lead-acid Battery
A lead-acid battery consists of a negative electrode made of spongy or porous lead. The lead is porous to facilitate the formation and dissolution of lead. The positive electrode consists of lead oxide. Both electrodes are immersed in an electrolytic solution of sulfuric acid and water. In case the electrodes come into contact with each other through the physical movement of the battery or changes in the thickness of the electrodes, an electrically insulating but chemically permeable membrane separates the two electrodes. This membrane also prevents electrical shorting through the electrolyte. The nominal voltage of a cell is about 2V, but the voltage varies between approximately 1.75 V and 2.4V depending on the SoC and the charging or discharging current. Lead acid batteries can deliver high currents for short periods and have a high power density.
Lead-acid batteries can achieve quite a long lifetime of several years. However, voltage regulation is crucial here. Especially in cars as starter battery, this is often insufficiently accurate, so that only 2 to 4 years of use are typical. Drive batteries or storage batteries can achieve a lifetime of between 5 and 15 years, depending on quality and stress. But these batteries differ significantly from car batteries. When lead acid batteries are of the same capacity and size, but of different weights, the heavier battery usually lasts longer because the lead frames are stronger. The power rating when new is not directly affected by this, as a weaker lead structure can also be designed with a large active surface area. The aging of lead acid batteries is mainly caused by internal corrosion of the lead structure of the electrodes, the formation of fine short circuits, and by sulfating of the lead.
Types of Lead-acid Batteries
We can find 2 main groups of lead-acid batteries:
- VLA battery (vented lead-acid battery) is a flooded or ventilated electrolyte lead-acid battery, where the electrodes are submerged in excess of liquid electrolyte. In the vented lead-acid batteries (VLA), there are 3 groups:
- Traction or deep cycle: These types of batteries are designed to produce a constant and small discharge for long periods of time. They are much less susceptible to degradation due to cycling and are required for applications where the batteries are regularly discharged, such as photovoltaic systems and electric vehicles.
- SLI battery: The term SLI refers to starting, lighting, and ignition. SLI batteries are designed to deliver the maximum current in a short space of time, keeping the voltage constant, therefore, they have a very low internal resistance. These batteries have a good life under shallow-cycle conditions but have a very poor lifetime under deep cycling (around 12-15 cycles).
- VRLA battery (valve-regulated lead-acid battery) is sealed or regulated by a valve where the electrolyte is immobilized in an absorbent separator or in a gel. There are two primary types of VRLA batteries:
- Absorbent glass mat (AGM). AGM lead-acid battery is a type of valve-regulated lead acid (VRLA) battery that has small gas channels in the electrolyte. Absorbed glass mat batteries lead acid battery is one of the lead acid technologies widely used for those applications because of its increased power and energy density and longer cycle life than regular flooded and maintenance free type lead acid batteries.
- Gel cell (gel battery). A modern gel battery is a VRLA battery with a gelated electrolyte. Gel batteries reduce the electrolyte evaporation and spillage (and subsequent corrosion problems) common to the wet-cell battery and boast greater resistance to shock and vibration.
Chemistry of Lead-acid Batteries – How it works
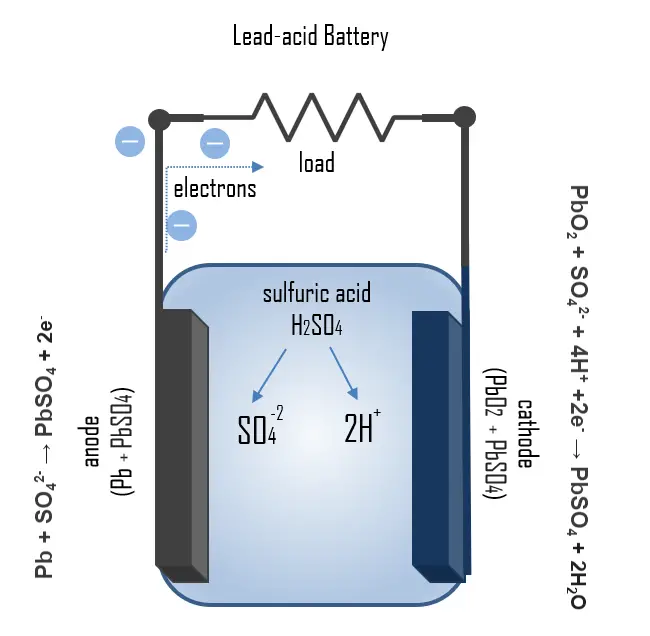
The principle of operation of the lead-acid battery can be illustrated by the chemical processes that take place during charging and discharging. During discharge, the process
Pb + SO42- → PbSO4 + 2e–
takes place at the anode. Lead is oxidized with the electrolyte to lead sulfate, releasing two electrons. Lead sulfate is also formed at the cathode by:
PbO2 + SO42- + 4H+ +2e– → PbSO4 + 2H2O
But in this reaction, a reduction of lead oxide takes place. Formed lead sulfate deposits as a coating on the electrodes and, to some extent also on the bottom of the housing. Since sulfuric acid is utilized during the discharge process, the SoC can be determined by measuring the density of the electrolyte.
During charging, the processes take place in the opposite direction so that the lead sulfate formed during discharging is oxidized to lead and reduced lead oxide, respectively. If the lead sulfate is completely consumed and the changing process is not stopped, electrolysis of the electrolyte begins. Overcharging with high charging voltages generates oxygen and hydrogen gas by electrolysis of water, which bubbles out and is lost. Sealed batteries have catalysts (Pd, Pt) above the vent where oxyhydrogen gas can recombine to water.
The resulting cell voltage can be determined from the galvanic series.
The total voltage of the redox reaction is thus:
E0 = 1.68V – ( – 0.36V) = 2.04V.
Advantages and Disadvantages of Lead-acid Batteries
Advantages:
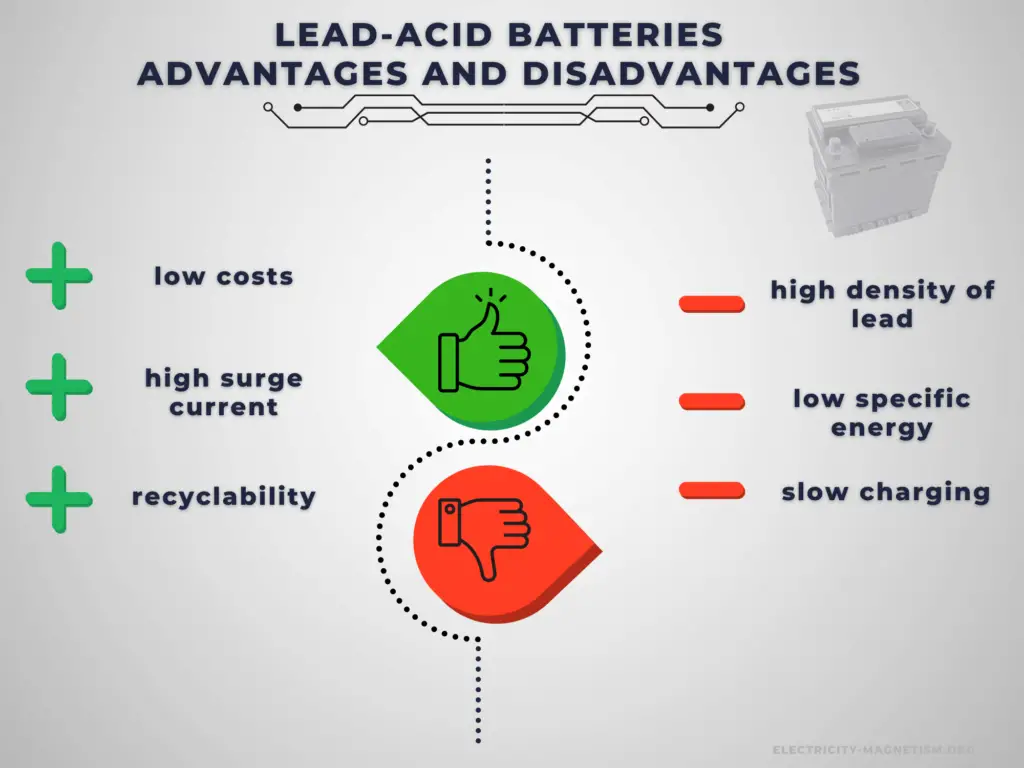
The lead–acid battery is relatively heavy for the amount of electrical energy it can supply. Its low manufacturing cost and its high surge current levels make it common where its capacity (over approximately 10 Ah) is more important than weight and handling issues.
Compared to modern rechargeable batteries, lead–acid batteries have relatively low energy density. Despite this, their ability to supply high surge currents means that the cells have a relatively large power-to-weight ratio. It has become the standard battery for the automotive industry and is commonly used as the power source for starting, lighting, and ignition (SLI) in cars, where it is able to deliver a very high peak current (let’s say about 450 amperes).
Disadvantages:
The disadvantage of this battery chemistry is that it is very sensitive to deep cycling compared to other battery systems, and due to the high density of lead, the specific energy of the batteries is quite low. Charging a lead acid battery system is slow, and it can take up to 16 hours for a full charge. It also requires a current and voltage limiting algorithm, hence limiting the charging current and power. If the charging current is too high, it will cause sulfation which may degrade the performance and cycle life of the battery. Furthermore, the charging voltage must be regulated as the maximum voltage applied is limited by the ambient temperature due to the risk of hydrogen gas generation.
Characteristics of Lead-acid Batteries
To compare and understand the capability of each battery, some important parameters are characteristic of each battery, also within a type of battery. These parameters are a reference when a battery is needed, and specific qualities are required since batteries are used in all types of devices and for infinite purposes.
Cell Voltage
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
A common voltage for automobile batteries is 12 volts (DC). But this battery consists of six 2V lead cells.
Cut-off Voltage
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
The truth is that any lead acid battery, be it a Gel Cell, AGM or flooded batteries, should be cut off at 11.6 volts.
Capacity
The coulometric capacity is the total Amp-hours available when the battery is discharged at a certain discharge current from 100% SOC to the cut-off voltage.
A common voltage for automobile batteries is 12 volts (DC). But this battery consists of six 2V lead cells. An average automotive battery might have a capacity of about 70 Ah, specified at a current of 3.5 A.
C-rate of Battery
C-rate is used to express how fast a battery is discharged or charged relative to its maximum capacity. It has units h−1. A 1C rate means that the discharge current will discharge the entire battery in 1 hour.
Self-discharge
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied.
A lead acid battery left in storage at moderate temperatures has an estimated self-discharge rate of 5% per month. This rate increases as temperatures rise and the risk of sulfation increases.
Degradation
Some degradation of rechargeable batteries occurs on each charge–discharge cycle. Degradation usually occurs because electrolyte migrates away from the electrodes or because active material detaches from the electrodes.
Deep cycle batteries can provide 200 to more than 3000 discharge/charge cycles. Starting batteries are not designed for sustained discharge and will last for only 50-60 use cycles.
Depth of Discharge
Depth of discharge is a measure of how much energy has been withdrawn from a battery and is expressed as a percentage of full capacity. For example, a 100 Ah battery from which 40 Ah has been withdrawn has undergone a 40% depth of discharge (DOD).
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery
Frequently asked questions
There are two basic types of batteries: primary and secondary. Primary batteries are “single use” and cannot be recharged. Dry cells and (most) alkaline batteries are examples of primary batteries. The second type is rechargeable and is called a secondary battery.
We can find 4 main types of lead-acid batteries: SLI battery, Traction battery, AGM battery and gel battery.
The lead–acid battery is relatively heavy for the amount of electrical energy it can supply. Its low manufacturing cost and its high surge current levels make it common where its capacity (over approximately 10 Ah) is more important than weight and handling issues. The disadvantage of this battery chemistry is that it is very sensitive to deep cycling compared to other battery systems, and due to the high density of lead, the specific energy of the batteries is quite low.








