30-second summary
Silver-oxide Battery
A silver-oxide battery is a primary cell using silver oxide as the cathode material and zinc for the anode. They are available in small sizes as button cells.
These cells maintain a nearly constant nominal voltage during discharge until fully depleted.
- The open circuit voltage of silver oxide batteries is 1.6 volts.
- The operating voltage at typical current drains is 1.55 volts or more.
- A typical silver-oxide battery in the standard SR721SW has about 25 mAh.
- A typical silver-oxide battery has a cutoff voltage of 1.2V.
- A typical annual self-discharge rate of 2-10% when being stored @20-25°C.
- Silver-oxide batteries feature a shelf life of 2-5 years.

An electric battery is essentially a source of DC electrical energy. It converts stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. A typical battery consists of one or more voltaic cells.
The fundamental principle in an electrochemical cell is spontaneous redox reactions in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated.
Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals. Even though a wide range of types of batteries exists with different combinations of materials, all of them use the same principle of the oxidation-reduction reaction. Batteries are designed so that the energetically favorable redox reaction can occur only when electrons move through the external part of the circuit.
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction. Because most of the resulting voltages are around 2V, cells are connected in series to obtain more practical electrical potentials (i.e. six 2V lead acid cells are connected in series to obtain a typical 12V battery).
Silver-oxide Battery
A silver-oxide battery is a primary cell using silver oxide as the cathode material, zinc for the anode, and a highly alkaline electrolyte consisting of either sodium hydroxide or potassium hydroxide. They are available in small sizes as button cells. The first letter in the IEC standard system identifies the chemical composition of the battery. S is for silver-oxide battery (as SR516). These cells maintain a nearly constant nominal voltage during discharge until fully depleted. A silver-oxide battery and a zinc-silver battery are different types of batteries.
The open circuit voltage of silver oxide batteries is 1.6 volts. The operating voltage at typical current drains is 1.55 volts or more. A typical silver-oxide battery in the standard SR721SW has about 25 mAh.
Advantages and Disadvantages of Silver-oxide Batteries
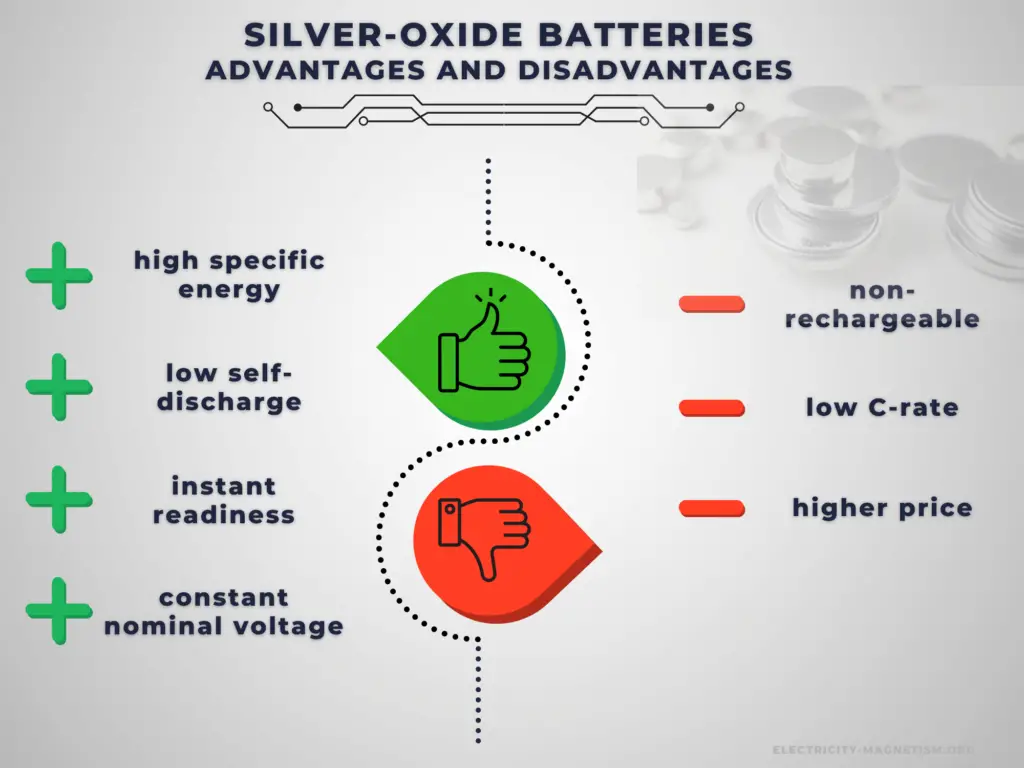
Advantages:
Primary batteries have higher energy density than rechargeable secondary cells. High specific energy, long storage times (low self-discharge), and instant readiness give primary batteries a unique advantage over other power sources. They are usually the best choice for low-drain applications. They can be carried to remote locations and used instantly, even after long storage; they are also readily available and environmentally friendly when disposed.
Disadvantages:
The main disadvantage of primary batteries is that they are non-rechargeable. Another disadvantage is their low C-rate. Even high current types are considered low in comparison to rechargeable batteries. They are also less environment friendly than rechargeable batteries. The application of primary batteries leads to a large amount of waste batteries to be recycled. For large batteries, primary batteries are usually not cost-effective.
Characteristics of Silver-oxide Batteries
To compare and understand the capability of each battery, some important parameters are characteristic of each battery, also within a type of battery. These parameters are a reference when a battery is needed, and specific qualities are required since batteries are used in all types of devices and for infinite purposes.
Cell Voltage
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
The open circuit voltage of silver oxide batteries is 1.6 volts. The operating voltage at typical current drains is 1.55 volts or more.
Cut-off Voltage
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
A typical silver-oxide battery has a cutoff voltage of 1.2V.
Capacity
The coulometric capacity is the total Amp-hours available when the battery is discharged at a certain discharge current from 100% SOC to the cut-off voltage.
A typical silver-oxide battery in the standard SR721SW has about 25 mAh.
C-rate of Battery
C-rate is used to express how fast a battery is discharged or charged relative to its maximum capacity. It has units h−1. A 1C rate means that the discharge current will discharge the entire battery in 1 hour.
Self-discharge
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied.
A typical annual self-discharge rate of 2-10% when being stored @20-25°C.
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery






