
An absorbent glass mat battery(AGM) is a type of valve-regulated lead acid (VRLA) battery that has small gas channels in the electrolyte. AGM batteries feature fiberglass mesh between the battery plates which serves to contain the electrolyte and separate the plates. The oxygen generated in the positive electrode during overcharging or float-charging can be transported to the negative electrode and reused. This process of recombination is called a closed oxygen cycle (COC) which can substantially reduce water loss since the water consumed for side reactions during charging can be compensated by regeneration from the reaction of hydrogen and oxygen. As a result, it becomes maintenance-free. AGM batteries are more resistant to self-discharging than conventional batteries within a wide range of temperatures. As with lead–acid batteries, in order to maximize the life of an AGM battery, it is important to follow the manufacturers charging specifications, and the use of a voltage-regulated charger is recommended. Absorbed glass mat batteries lead acid battery is one of the lead acid technologies widely used for those applications because of its increased power and energy density and longer cycle life than regular flooded and maintenance free type lead acid batteries.
Other Types of Lead-acid Batteries
We can find 2 main groups of lead-acid batteries:
- VLA battery (vented lead-acid battery) is a flooded or ventilated electrolyte lead-acid battery, where the electrodes are submerged in excess of liquid electrolyte. In the vented lead-acid batteries (VLA), there are 3 groups:
- Traction or deep cycle: These types of batteries are designed to produce a constant and small discharge for long periods of time. They are much less susceptible to degradation due to cycling and are required for applications where the batteries are regularly discharged, such as photovoltaic systems and electric vehicles.
- SLI battery: The term SLI refers to starting, lighting, and ignition. SLI batteries are designed to deliver the maximum current in a short space of time, keeping the voltage constant, therefore, they have a very low internal resistance. These batteries have a good life under shallow-cycle conditions but have a very poor lifetime under deep cycling (around 12-15 cycles).
- VRLA battery (valve-regulated lead-acid battery) is sealed or regulated by a valve where the electrolyte is immobilized in an absorbent separator or in a gel. There are two primary types of VRLA batteries:
- Absorbent glass mat (AGM). AGM lead-acid battery is a type of valve-regulated lead acid (VRLA) battery that has small gas channels in the electrolyte. Absorbed glass mat batteries lead acid battery is one of the lead acid technologies widely used for those applications because of its increased power and energy density and longer cycle life than regular flooded and maintenance free type lead acid batteries.
- Gel cell (gel battery). A modern gel battery is a VRLA battery with a gelated electrolyte. Gel batteries reduce the electrolyte evaporation and spillage (and subsequent corrosion problems) common to the wet-cell battery and boast greater resistance to shock and vibration.
Chemistry of Lead-acid Batteries – How it works
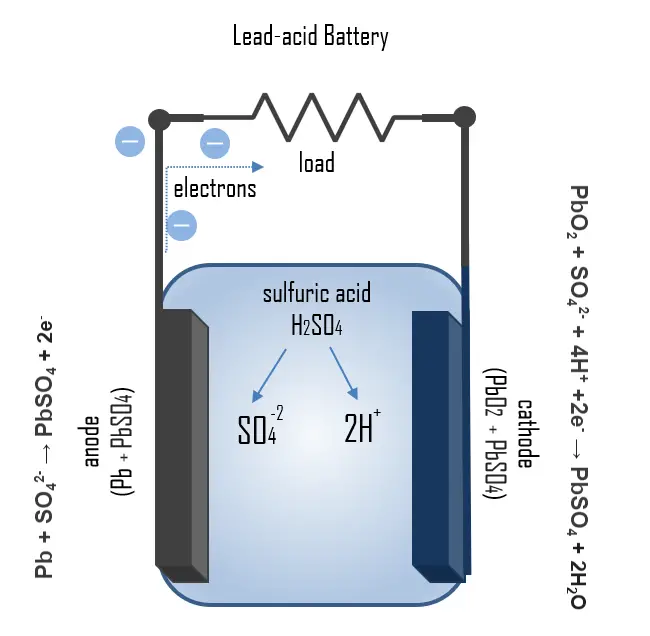
The principle of operation of the lead-acid battery can be illustrated by the chemical processes that take place during charging and discharging. During discharge, the process
Pb + SO42- → PbSO4 + 2e–
takes place at the anode. Lead is oxidized with the electrolyte to lead sulfate, releasing two electrons. Lead sulfate is also formed at the cathode by:
PbO2 + SO42- + 4H+ +2e– → PbSO4 + 2H2O
But in this reaction, a reduction of lead oxide takes place. Formed lead sulfate deposits as a coating on the electrodes and, to some extent also on the bottom of the housing. Since sulfuric acid is utilized during the discharge process, the SoC can be determined by measuring the density of the electrolyte.
During charging, the processes take place in the opposite direction so that the lead sulfate formed during discharging is oxidized to lead and reduced lead oxide, respectively. If the lead sulfate is completely consumed and the changing process is not stopped, electrolysis of the electrolyte begins. Overcharging with high charging voltages generates oxygen and hydrogen gas by electrolysis of water, which bubbles out and is lost. Sealed batteries have catalysts (Pd, Pt) above the vent where oxyhydrogen gas can recombine to water.
The resulting cell voltage can be determined from the galvanic series.
The total voltage of the redox reaction is thus:
E0 = 1.68V – ( – 0.36V) = 2.04V.

