30-second summary
AA Battery
An AA battery is one of the most common types of single cell cylindrical batteries. AA batteries are common in portable electronic devices.
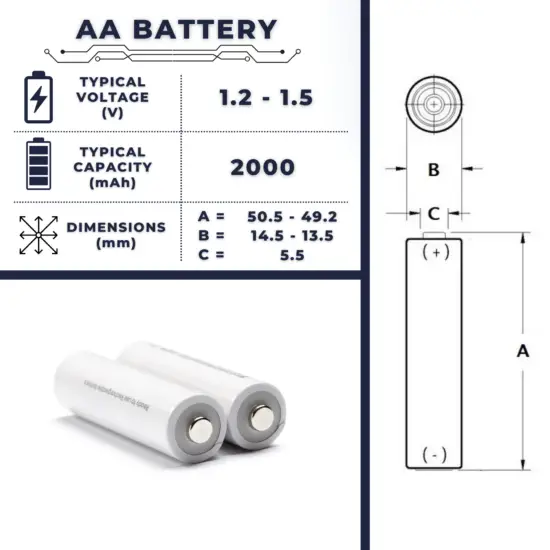
Several different chemistries are used in their construction. The exact terminal voltage, capacity and practical discharge rates depend on cell chemistry; however, devices designed for AA cells will usually only take 1.2-1.5 V unless specified by the manufacturer.
Types of AA Batteries:
The AA battery is one of the most common types of single-cell cylindrical batteries. AA batteries are common in portable electronic devices.
Characteristics of AA Batteries
To compare and understand the capability of each battery, some important parameters are characteristic of each battery, also within a type of battery. These parameters are a reference when a battery is needed, and specific qualities are required since batteries are used in all types of devices and for infinite purposes.
Cell Voltage
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
Alkaline batteries have an open cell voltage of about 1.5 V. A common open circuit voltage for NiMH batteries (e.g. AAA and AA) is 1.2V.
Cut-off Voltage
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
When testing the capacity of a NiMH or NiCd battery, a cut-off voltage of 1.0 V per cell is normally used, whereas 0.9 V is normally used as the cut-off voltage of an alkaline cell.
Capacity
The coulometric capacity is the total Amp-hours available when the battery is discharged at a certain discharge current from 100% SOC to the cut-off voltage.
A typical alkaline or NiMH battery in the standard “AA” size has about 2000 to 3000 mAh (or 2 to 3 Ah).
C-rate of Battery
C-rate is used to express how fast a battery is discharged or charged relative to its maximum capacity. It has units h−1. A 1C rate means that the discharge current will discharge the entire battery in 1 hour.
To obtain a reasonably good capacity reading, manufacturers commonly rate alkaline and lead acid batteries at a very low 0.05C, or a 20-hour discharge.
Self-discharge
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied.
One of the main advantages of alkaline batteries is that they are easy to store. They are chemically stable, and they have a very low self-discharge rate. Alkaline batteries typically lose 2 to 3 percent of their original charge per year when stored at room temperature (20–30 °C). The NiMH battery also has high self-discharge and can lose up to 20 % of its charge during the first 24 hours and thereafter 10 % per month.
Degradation
Some degradation of rechargeable batteries occurs on each charge-discharge cycle. Degradation usually occurs because electrolyte migrates away from the electrodes or because active material detaches from the electrodes.
The cycle life for NiMH batteries is typically 700-1,000 life cycles.
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery







