30-second summary
Lithium Polymer Battery
A lithium-ion battery, also known as the Li-ion battery, is a type of secondary (rechargeable) battery composed of cells in which lithium ions move from the anode through an electrolyte to the cathode during discharge and back when charging.
A lithium-ion polymer (LiPo) battery (also known as Li-pol, lithium-poly, and other names) is a type of Li-ion battery with a polymer electrolyte instead of a liquid electrolyte. All LiPo batteries use a high-conductivity gel polymer as the electrolyte. Lithium polymer cells have evolved from lithium-ion and lithium-metal batteries.
Lithium-ion batteries are used in many laptop computer batteries, cordless power tools, certain electric cars, electric kick scooters, most e-bikes, portable power banks, and LED flashlights.
The overall reaction during discharge is:
C6Li + CoO2 ⇄ C6 + LiCoO2

Lithium Polymer Battery
A lithium-ion battery, also known as the Li-ion battery, is a type of secondary (rechargeable) battery composed of cells in which lithium ions move from the anode through an electrolyte to the cathode during discharge and back when charging.
A lithium-ion polymer (LiPo) battery (also known as Li-pol, lithium-poly, and other names) is a type of Li-ion battery with a polymer electrolyte instead of a liquid electrolyte. All LiPo batteries use a high-conductivity gel polymer as the electrolyte. Lithium polymer cells have evolved from lithium-ion and lithium-metal batteries. The primary difference between lithium-ion and Li-pol is that instead of using a liquid lithium-salt electrolyte (such as LiPF6) held in an organic solvent, the battery uses a solid polymer electrolyte (SPE) such as polyethylene oxide (PEO), polyacrylonitrile (PAN), polymethyl methacrylate (PMMA) or polyvinylidene fluoride (PVdF). LiPos provide higher specific energies than other lithium batteries, often used in systems where weight is an important factor, such as mobile devices, drones, and some electric vehicles.
The cathode is made of a composite material (an intercalated lithium compound) and defines the name of the Li-ion battery cell. The anode is usually made out of porous lithiated graphite. The electrolyte can be liquid, polymer, or solid. The separator is porous to enable the transport of lithium ions and prevents the cell from short-circuiting and thermal runaway.
Chemistry, performance, cost, and safety characteristics vary across types of lithium-ion batteries. Handheld electronics mostly use lithium polymer batteries (with a polymer gel as electrolyte), a lithium cobalt oxide (LiCoO2) cathode material, and a graphite anode, which offer high energy density.
Li-ion batteries, in general, have a high energy density, no memory effect, and low self-discharge. One of the most common types of cells is 18650 battery, which is used in many laptop computer batteries, cordless power tools, certain electric cars, electric kick scooters, most e-bikes, portable power banks, and LED flashlights. The nominal voltage is 3.7 V.
Note that non-rechargeable primary lithium batteries (like lithium button cells CR2032 3V) must be distinguished from secondary lithium-ion or lithium-polymer, which are rechargeable batteries. Primary lithium batteries contain metallic lithium, which lithium-ion batteries do not.
Composition of Lithium Polymer Battery
A typical lithium-ion cell contains:
- Cathode: The cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction. In the case of lithium batteries, cathode materials are generally constructed from LiCoO2 or LiMn2O4. For the cathode, it is important to hold a large amount of lithium without significant change in structure, have good chemical and electrochemical stability with electrolyte, be a good electrical conductor and diffuser of lithium ions, and be of low cost.
- Anode: The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during an electrochemical reaction. One of the most common anode materials used today is lithiated graphite, LixC6, which is composed of graphite sheets intercalated with lithium. New materials, such as those based on Silicon and other elemental blends, are being researched. Lithiated graphite has a unit cell with an HCP structure.
- Separator. A separator is a permeable membrane placed between a battery’s anode and cathode. The main function of a separator is to keep the two electrodes apart to prevent electrical short circuits while also allowing the transport of ionic charge carriers that are needed to close the circuit during the passage of current in an electrochemical cell. Commercially available liquid electrolyte cells use microporous polyolefin materials, such as polyethylene (PE) or polypropylene (PP). Separators in Li-ions have to be electrochemically and chemically stable relative to the electrolyte and electrode materials. Functional separators that use MOF-coated membranes to perform the dual functions of the electrolyte and separator are being developed to support the design of high-performance Li-metal batteries for high-energy systems in electric vehicles and electric aircraft.
- Electrolyte: The choice of electrolyte in all batteries is critical for performance as well as safety. Most of the electrolytes used in commercial lithium-ion batteries are non-aqueous solutions, in which lithium hexafluorophosphate (LiPF6) salt dissolved in organic carbonates, in particular, mixtures of ethylene carbonate (EC) with dimethyl carbonate (DMC), propylene carbonate (PC), diethyl carbonate (DEC), and/or ethyl methyl carbonate (EMC). A good electrolyte must have low reactivity with other cell components, high ionic conductivity, low toxicity, a large window of electrochemical voltage stability (0-5V), and be thermally stable.
Applications of Lithium Polymer Batteries
The most common Li-ion type in small consumer electronics (laptops-cell phones) is lithium-cobalt-oxide (LCO) due to its high specific energy. Tesla Motors uses laptop-sized LCO cells in their EVs combined with a liquid cooling system safety issues, but low specific power and life span prevent this type to be a good choice for EVs.

On the other hand, lithium-iron-phosphate (LiFePO4/LFP) does not experience thermal runaway and has almost no fire hazards since no oxygen is released at high temperatures. LiFePO4 cells have a good life span, low costs per Ah and kW, good power capabilities, and are extremely safe, but the specific energy is low, and the performance is poor at low temperatures.
See also: Lithium-ion Batteries and Grid Energy Storage
Types of Lithium-ion Batteries
The cathode is made of a composite material (an intercalated lithium compound) and defines the name of the Li-ion battery cell. There are two kinds of electrodes: intercalation and conversion electrodes. The intercalation electrodes are materials that function as host materials where lithium ions can intercalate. A typical example is LiCoO2 and its derivatives. Conversion-type cathode materials are some of the key candidates for the next generation of rechargeable Li and Li-ion batteries.
One of the most common lithium batteries are
- Lithium Cobalt Oxide (LiCoO2). LiCoO2 is the most commonly used cathode material. LiCoO2 batteries have very stable capacities, although their capacities are lower than those based on nickel-cobalt-aluminum (NCA) oxides. However, cobalt is relatively expensive compared to other transition metals, such as manganese and iron, despite the attractive electrical properties of LiCoO2 cathodes. Currently, we can find this type of battery in mobile phones, tablets, laptops, and cameras.
- Lithium Manganese Oxide (LiMn2O4). LiMn2O4 is a promising cathode material with a cubic spinel structure. LiMn2O4 is one of the most studied manganese oxide-based cathodes because it contains inexpensive materials. A further advantage of this battery is enhanced safety and high thermal stability, but the cycle and calendar life is limited. This type of battery is found in power tools, medical devices, and powertrains.
- Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2) – NMC. Nickel manganese cobalt (NMC) batteries contain a cathode made of a combination of nickel, manganese, and cobalt. NMC is one of the most successful cathode combinations in Li-ion systems. It can be tailored to serve as energy cells or power cells like Li-manganese. NMC batteries are used for power tools, e-bikes, and other electric powertrains.
- Lithium Iron Phosphate (LiFePO4) – LFP. LiFePO4 is one of the most recent cathode materials to be introduced. As of 2017, LiFePO4 is a candidate for large-scale production of lithium-ion batteries, such as electric vehicle applications, due to its low cost, excellent safety, and high cycle durability. The energy density of an LFP battery is lower than that of other common lithium-ion battery types, such as Nickel Manganese Cobalt (NMC). Because of their lower cost, high safety, low toxicity, long cycle life, and other factors, LFP batteries are finding a number of roles in vehicle use, utility-scale stationary applications, and backup power. Working voltage = 3.0 ~ 3.3 V. Cycle life ranges from 2,700 to more than 10,000 cycles depending on conditions.
- Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2) – NCA. In 1999, Lithium nickel cobalt aluminum oxide battery, or NCA, appeared in some special applications, and it is similar to the NMC. It offers high specific energy, a long life span, and a reasonably good specific power. NCA’s usable charge storage capacity is about 180 to 200 mAh/g. The capacity of NCA is significantly higher than that of alternative materials such as LiCoO2 with 148 mAh/g, LiFePO4 with 165 mAh/g, and NMC 333 (LiNi0,33Mn0,33Co0,33O2)with 170 mAh/g. Voltage of these batteries is between 3.6 V and 4.0 V, at a nominal voltage of 3.6 V or 3.7 V. Another advantage of NCA is its excellent fast charging capability. Nevertheless, its weak points are the the limited resources of cobalt and nickel and the high cost.
Advantages and Disadvantages of Lithium Polymer Batteries
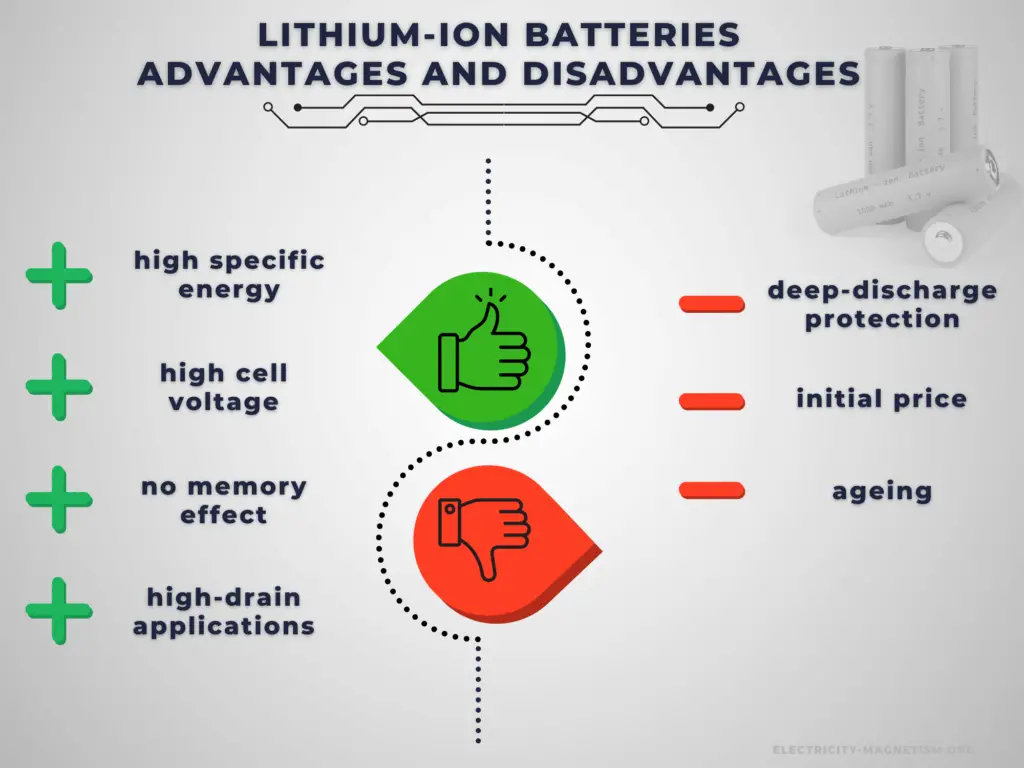
There are many advantages to using a Li-ion cell. As a result, the technology is being used increasingly for a huge number of widely varying applications. A lithium-ion battery offers advantages over other battery types in several areas.
Advantages:
The main advantage of rechargeable cells is that they may be recharged after discharge. Therefore, rechargeable batteries are more environmentally friendly than primary batteries. Not only can they be used repeatedly, but they generate less waste over the long term. This is particularly true in the case of power-intensive devices, which consume batteries at an increased rate. Another very important advantage is a high C-rate. Rechargeable cells have better power output capabilities compared to primary cells and are used for high-power applications.
There are several specific advantages to lithium-ion batteries.
- Cell voltage. Lithium-ion batteries have a high operating voltage of 3-5 volts, depending on the specific chemistry. This allows for an equivalent power operation at a lower current draw, and the battery will last longer on a single charge.
- High energy density. Lithium-ion batteries have a high energy density, so lithium-ion batteries are lightweight and compact. NCA’s usable charge storage capacity is about 180 to 200 mAh/g. The capacity of NCA is significantly higher than that of alternative materials such as LiCoO2 with 148 mAh/g, LiFePO4 with 165 mAh/g, and NMC 333 (LiNi0,33Mn0,33Co0,33O2)with 170 mAh/g.
- No memory effect. Unlike NiCd and older NiMH batteries, Li-ion batteries do not exhibit any memory effect and have long shelf lives – up to 5 years. Ni-Cd cells required a periodic discharge to ensure that they did not exhibit the memory effect.
- Low self-discharge. Li-ion rechargeable batteries have a self-discharge rate typically stated by manufacturers to be 1.5–2% per month. Old rechargeable batteries self-discharge more rapidly, especially nickel-based batteries. A freshly charged nickel-cadmium (Ni-Cd) battery loses 10% of its charge in the first 24 hours and thereafter discharges at a rate of about 10% a month.
- Load characteristics. The load characteristics of a lithium-ion cell or battery are reasonably good. They provide a reasonably constant 3.6 volts per cell before falling off as the last charge is used.
Disadvantages:
Battery price is one of the challenging factors in choosing the right rechargeable battery for your device or applications. It greatly affects the decision of the buyer. Rechargeable batteries have higher initial costs than their primary counterparts. Another important disadvantage is their self-discharge. In low-drain applications, the service life is more important, and the self-discharge characteristics of a rechargeable battery mean that they are less suitable for use as the primary energy source.
There are several specific disadvantages to lithium-ion batteries.
- An electronic battery management system is required. Lithium-ion batteries use monitoring electronics to ensure over-charge and deep-discharge protection.
- A thermal management system is required. Batteries generate heat when being charged or discharged, especially at high currents. Large battery packs, such as those used in electric vehicles, are generally equipped with thermal management systems that maintain a temperature between 15 °C (59 °F) and 35 °C.
- Ageing. One of the major lithium-ion battery disadvantages for consumer electronics is that lithium-ion batteries suffer from ageing. Not only is this time or calendar dependent, but it is also dependent upon the number of charge-discharge cycles that the battery has undergone. Most modern 18650 lithium-ion batteries, which are common for laptop batteries, have a typical cycle life of 300 – 500 (charge, discharge cycles). When in C-rate or high DOD situations, this can decrease substantially to 200 cycles.
- Higher cost. A major lithium-ion battery disadvantage is its cost. Typically they are around 40% more costly to manufacture than NiMH cells.
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery
Frequently asked questions
Lithium metal batteries are non-rechargeable batteries, typically button cells. Lithium-ion batteries are widely used rechargeable batteries.
Li-ion batteries are typically used in many laptop computer batteries, cordless power tools, certain electric cars, electric kick scooters, most e-bikes, portable power banks, and LED flashlights.
There are several specific advantages to lithium-ion batteries. The most important advantages are their high cell voltage, high energy density, and no memory effect. On the other hand, they are expensive and must use monitoring electronics to ensure over-charge and deep-discharge protection.
Yes, there are. You can use 14500 lithium cells for AA cells, but remember they are NOT the same voltage. AA dry cells, alkaline, and NiMH are 1.2 to 1.5 V. Lithium Ion are 3.6V. LIFEPO4 cells are 3.3V. So forget it if you want to replace a single AA cell. If you want to replace 2–3 AA, you will need a “spacer” fake battery.


