A primary battery or primary cell is a non-rechargeable battery that is designed to be used once discarded after use. This means that the redox reaction within the cell is not reversible like in a secondary (rechargeable) battery. The primary alkaline battery is a widely used product, which is essential for powering many portable devices, such as power tools, radios, toys, and remote controls. These batteries are most commonly used in portable devices with low current drains, are used only intermittently, or are used well away from an alternative power source, such as in alarm and communication circuits where other electric power is only intermittently available.
Advantages and Disadvantages of Primary Batteries
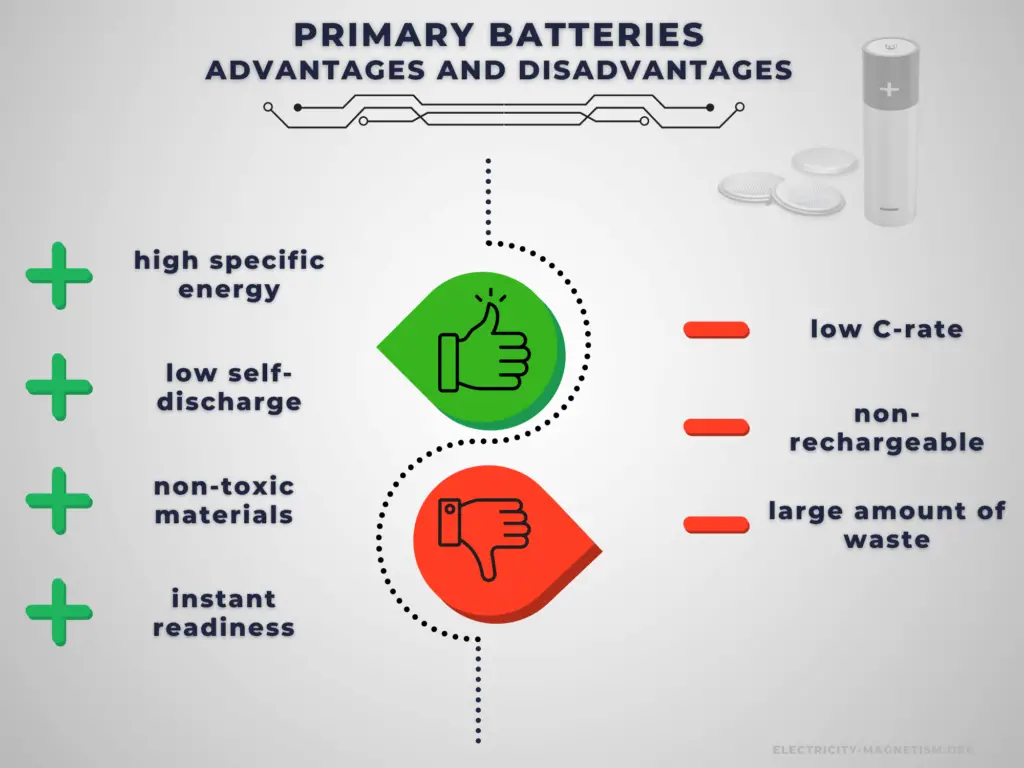
Advantages:
Primary cells have higher energy density than rechargeable secondary cells. High specific energy, long storage times (low self-discharge), and instant readiness give primary batteries a unique advantage over other power sources. They are usually the best choice for low-drain applications. They can be carried to remote locations and used instantly, even after long storage; they are also readily available and environmentally friendly when disposed.
Disadvantages:
The main disadvantage of primary batteries is that they are non-rechargeable. Another disadvantage is their low C-rate. Even high current types are considered low in comparison to rechargeable batteries. They are also less environment friendly than rechargeable batteries. The application of primary batteries leads to a large amount of waste batteries to be recycled. For large batteries, primary batteries are usually not cost-effective.
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery



