30-second summary
Electric Polarization
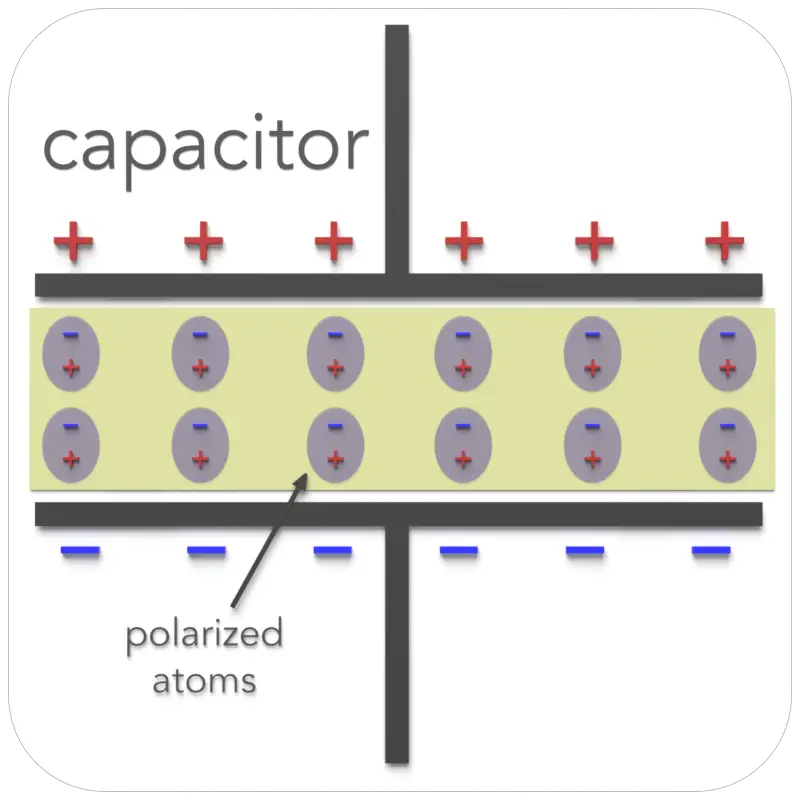
Electric polarization is a slight relative shift of positive and negative electric charge in opposite directions within atoms or molecules of an insulator, or dielectric, induced by an external electric field.
The polarization of the dielectric by the applied electric field increases the capacitor’s surface charge for the given electric field strength. The application of an electric field between the two plates induces some opposite charge on the dielectric, which opposes the applied electric field. The net result is that the electric field inside the dielectric (which fills the area between the plates) is reduced, allowing the capacitor to store more charge.
There are three types of polarization:
- Electronic polarization.
- Orientational polarization.
- Ionic polarization.
See also: Capacitance
See also: Electric polarization
See also: Permittivity
Electric Polarization
In contrast to metals, where charges are free to move throughout the material, in dielectrics, all the charges are attached to specific atoms and molecules. These charges are known as bound charges. These charges are able, however, can be displaced (polarized) within an atom or a molecule by an application of an electric field.
Electric polarization is a slight relative shift of positive and negative electric charge in opposite directions within atoms or molecules of an insulator, or dielectric, induced by an external electric field.
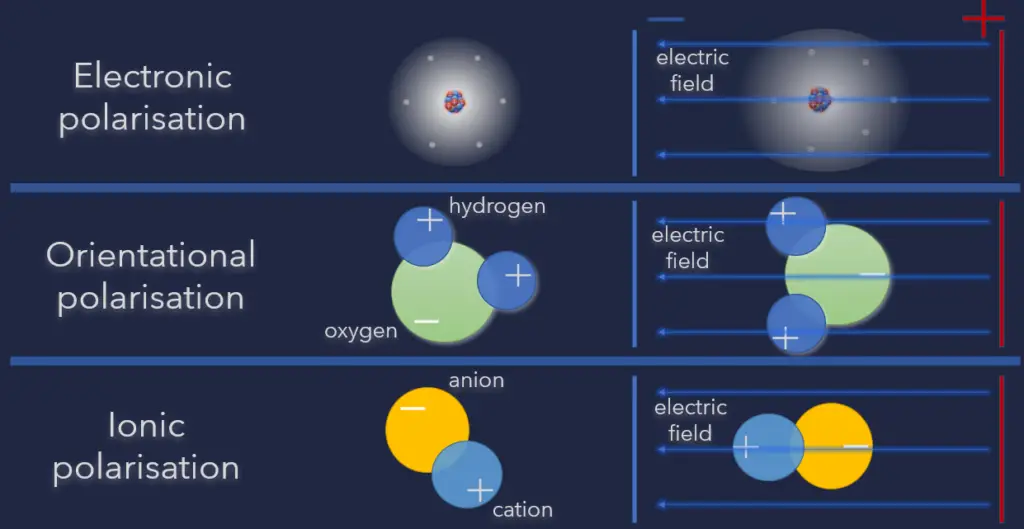
These microscopic displacements are not as dramatic as the rearrangement of charge in a conductor, but their cumulative effects account for the characteristic behavior of dielectric materials. When an external electric field is applied to a dielectric material, this material becomes polarized, which means that it acquires a dipole moment. This property of dielectrics is known as polarizability. When an electric field acts on a molecule, the positive charges are displaced along the field, while the negative charges are displaced in a direction opposite to that of the field. The effect is, therefore, to pull the opposite charges apart, i.e., to polarize the molecule. There are three types of polarization:
- Electronic polarization. Here when the external field is applied, the electron clouds of atoms are displaced with respect to the heavy nuclei within the dimensions of these atoms. This is called electronic polarization.
- Orientational polarization. Orientational polarization is a polarization that is either inherent to molecules, or can be induced in any molecule in which the asymmetric distortion of the nuclei is possible (distortion polarization). Polar molecules are those types of dielectrics in which the chances of positive and negative molecules colliding are nil or zero. This is because they are all asymmetrical in form. H2O is a typical example. When there is no electric field, the electric dipole moment of these molecules moves in an unpredictable direction. The average dipole moment is 0 as a result of this. If there is an external electric field, the molecules will assemble in the same direction as the electric field.
- Ionic polarization. Ionic polarization is polarization caused by relative displacements between positive and negative ions in ionic crystals (for example, NaCl).
Dielectric Constant
Third, experimentally, it was found that capacitance C increases when the space between the conductors is filled with dielectrics. The polarization of the dielectric by the applied electric field increases the capacitor’s surface charge for the given electric field strength. The application of an electric field between the two plates induces some opposite charge on the dielectric, which opposes the applied electric field. The net result is that the electric field inside the dielectric (which fills the area between the plates) is reduced, allowing the capacitor to store more charge.
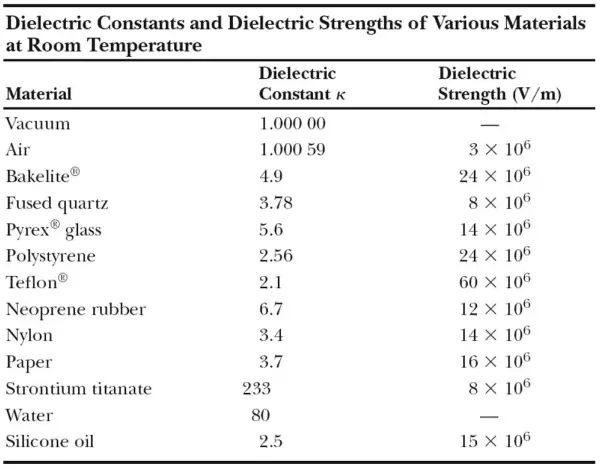
The dielectric constant of an insulator measures the ability of the insulator or dielectric to store electric energy in an electrical field. The dielectric constant, which is denoted by κ (kappa), is the same quantity as the relative permittivity denoted by εr . The dielectric constant is still used but deprecated by standards organizations in engineering.
The dielectric constant of a vacuum is unity by definition. Because air is mostly empty space, its measured dielectric constant is only slightly greater than unity. Even common paper can significantly increase the capacitance of a capacitor, and some materials, such as strontium titanate, can increase the capacitance by more than two orders of magnitude.
In a region completely filled by a dielectric material of dielectric constant κe, all electrostatic equations containing the permittivity constant ε0 are to be modified by replacing ε0 with κeε0 .
The greater the dielectric constant, the greater the amount of charge that can be held. The capacitance of a capacitor is increased by a factor of the dielectric constant when the gap between the plates is completely filled with a dielectric.
C = εrC0 = κeC0
where C0 is the capacitance between the plates with no dielectric.