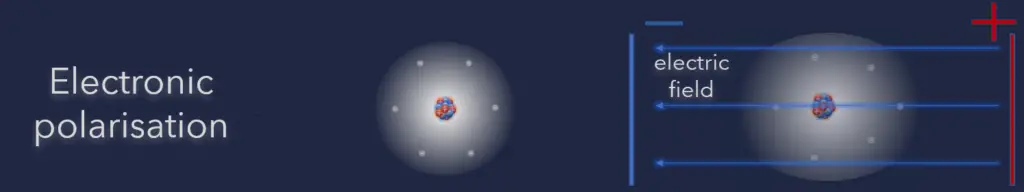
Electronic polarization is the redistribution of electrons in a molecule or material in response to an external electric field. In other words, when an electric field is applied, the negatively charged electrons within a material or molecule shift slightly in one direction, causing a temporary separation of charges and the creation of a dipole moment.
This effect is important in many areas of science, including chemistry, physics, and materials science. For example, electronic polarization plays a crucial role in the behavior of materials such as ferroelectric and piezoelectric materials, which have important technological applications in fields such as data storage and sensing. It is also important in the behavior of molecules in chemical reactions and in the properties of materials used in electronic devices.
Electric Polarization
In contrast to metals, where charges are free to move throughout the material, in dielectrics, all the charges are attached to specific atoms and molecules. These charges are known as bound charges. These charges are able, however, can be displaced (polarized) within an atom or a molecule by an application of an electric field.
Electric polarization is a slight relative shift of positive and negative electric charge in opposite directions within atoms or molecules of an insulator, or dielectric, induced by an external electric field.
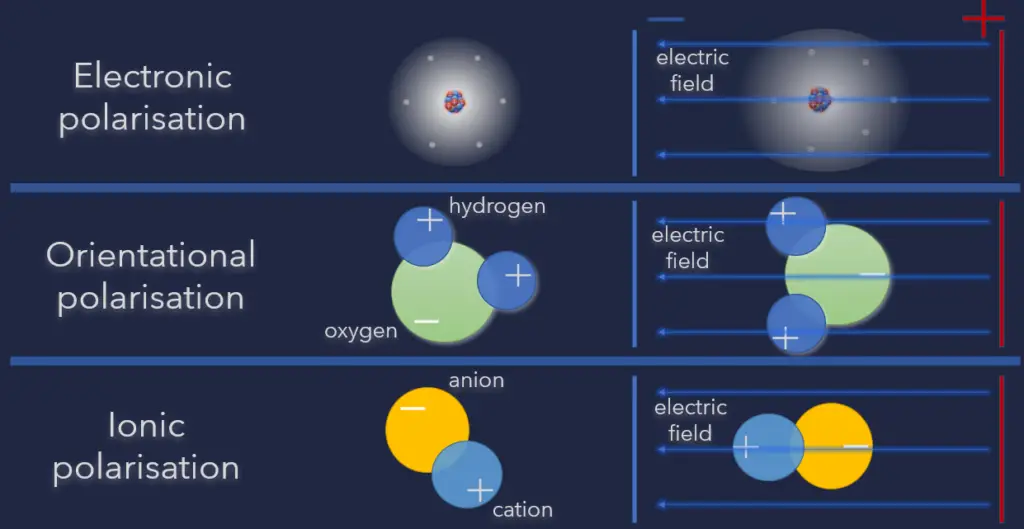
These microscopic displacements are not as dramatic as the rearrangement of charge in a conductor, but their cumulative effects account for the characteristic behavior of dielectric materials. When an external electric field is applied to a dielectric material, this material becomes polarized, which means that it acquires a dipole moment. This property of dielectrics is known as polarizability. When an electric field acts on a molecule, the positive charges are displaced along the field, while the negative charges are displaced in a direction opposite to that of the field. The effect is, therefore, to pull the opposite charges apart, i.e., to polarize the molecule. There are three types of polarization:
- Electronic polarization. Here when the external field is applied, the electron clouds of atoms are displaced with respect to the heavy nuclei within the dimensions of these atoms. This is called electronic polarization.
- Orientational polarization. Orientational polarization is a polarization that is either inherent to molecules, or can be induced in any molecule in which the asymmetric distortion of the nuclei is possible (distortion polarization). Polar molecules are those types of dielectrics in which the chances of positive and negative molecules colliding are nil or zero. This is because they are all asymmetrical in form. H2O is a typical example. When there is no electric field, the electric dipole moment of these molecules moves in an unpredictable direction. The average dipole moment is 0 as a result of this. If there is an external electric field, the molecules will assemble in the same direction as the electric field.
- Ionic polarization. Ionic polarization is polarization caused by relative displacements between positive and negative ions in ionic crystals (for example, NaCl).