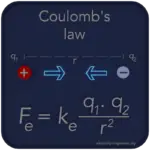
Coulomb’s Law describes the electrostatic force between charged particles, with the force dependent on the charges’ magnitudes and their distance.
Introduction to Coulomb’s Law
Coulomb’s Law is a fundamental principle in electrostatics that describes the force between two charged particles. Discovered by French physicist Charles-Augustin de Coulomb in 1785, the law quantifies the electrostatic interaction between charged objects and plays a vital role in understanding the behavior of charged particles and the electric field.
The Equation
Coulomb’s Law is mathematically expressed as:
F = k * |q1 * q2| / r2
Where:
F is the electrostatic force between two charged particles.
k is Coulomb’s constant (approximately 8.99 x 109 N m2 C-2).
q1 and q2 are the magnitudes of the charges in coulombs.
r is the distance between the centers of the charged particles in meters.
Force and Direction
The magnitude of the electrostatic force is directly proportional to the product of the charges and inversely proportional to the square of the distance between them. The force has both magnitude and direction:
Attraction: If the charges have opposite signs (one positive and one negative), the electrostatic force is attractive, pulling the particles toward each other.
Repulsion: If the charges have the same sign (both positive or both negative), the electrostatic force is repulsive, pushing the particles away from each other.
Limitations and Assumptions
Coulomb’s Law has certain limitations and assumptions:
Stationary Charges: The law is only valid for charges that are not moving or moving very slowly compared to the speed of light.
Point Charges: Coulomb’s Law assumes that charges are point-like, with no size or shape. In practice, this assumption is valid when the distance between the charges is much larger than their size.
Vacuum: The law assumes the charges are in a vacuum, with no other materials affecting the force. For materials with different permittivity, the force can be adjusted by including the relative permittivity (εr) in the equation.
Applications of Coulomb’s Law
Coulomb’s Law has various applications in science and technology:
Electric Field: The law is the basis for calculating the electric field generated by charged particles or systems of particles.
Electrostatics: It provides a foundation for understanding electrostatic interactions and their influence on the behavior of charged particles in various settings.
Physics and Chemistry: The law is used to analyze atomic and molecular interactions, including the forces between ions in ionic compounds or within molecules.