30-second summary
Coulomb’s Law – Equation
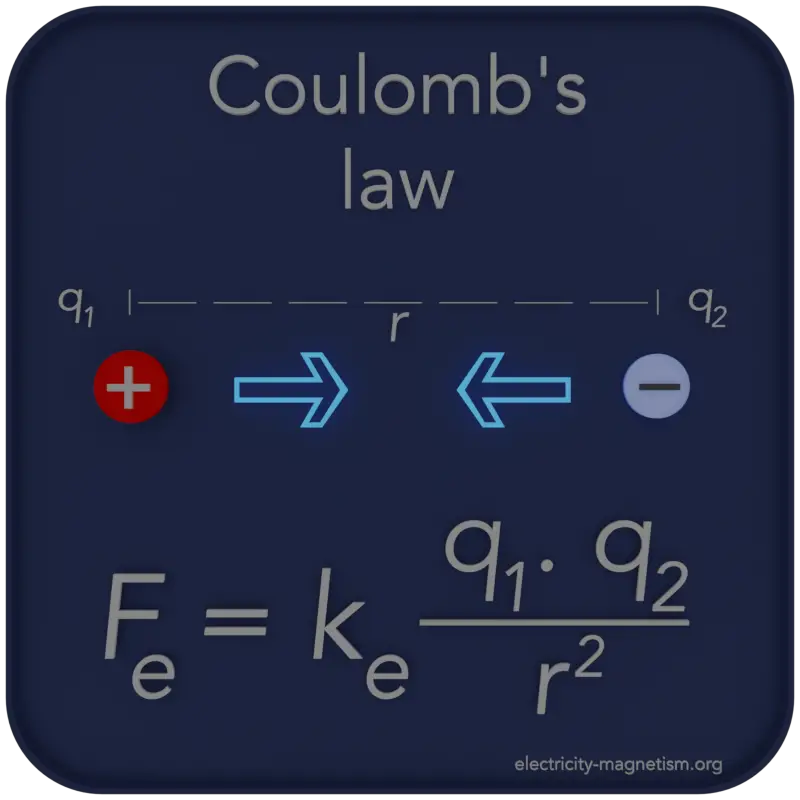
Coulomb’s law is a law of physics that describes the electric forces that act between electrically charged particles.
This is the scalar form of Coulomb’s law, which gives the magnitude of the vector of the electrostatic force F between two point charges, but not its direction. Here, K or ke is Coulomb’s constant (ke ≈ 8.988×109 N⋅m2⋅C−2), q1 and q2 are the signed magnitudes of the charges, and the scalar r is the distance between the charges.
Coulomb’s law may be expressed also by its vector form.
Coulomb’s Law Equation
Coulomb’s law can be used to calculate the force between charged particles (e.g., two protons) or between two charged objects. The magnitude of the electric force F is directly proportional to the amount of one electric charge, q1, multiplied by the other, q2, and inversely proportional to the square of the distance between the particles. Coulomb’s Law is stated as the following equation.
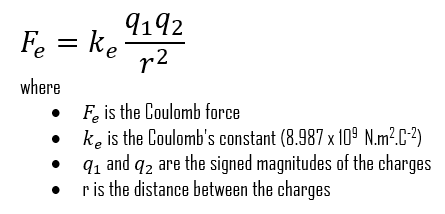
This is the scalar form of the Coulomb’s law, which gives the magnitude of the vector of the electrostatic force F between two point charges, but not its direction. Here, K or ke is Coulomb’s constant (ke ≈ 8.988×109 N⋅m2⋅C−2), q1 and q2 are the signed magnitudes of the charges, and the scalar r is the distance between the charges. The greater the charge on the objects, the greater the electrostatic field. The greater the distance between the objects, the weaker the electrostatic field between them, and vice versa. If q1 and q2 are both either positively or negatively charged, the force is repulsive. If q1 and q2 are opposite polarity or charge, the force is attractive. The proportionality of the electric force to 1/r2 has been verified with great precision.
Thus, 1C is that amount of charge which, if placed on each of two point objects that are 1.0 m apart, will result in each object exerting a force of:
(9.0×109 N-m2/C2)x(1.0C)x(1.0C)/(1.0m)2 = 9.0 x 109N
This is an enormous force. We rarely encounter charges as large as a coulomb. Charges produced by rubbing ordinary objects (such as a comb or plastic ruler) are typically around a microcoulomb (?C = 10-6 C) or less.
The same charges are repelled and the opposite charges are attracted. The base unit of electric charge is the (negative) charge of the electron, 1.602×10-19 coulombs. The electric force is operative between charges down to distances of at least 10-16 metre, or approximately one-tenth of the diameter of atomic nuclei.
Coulomb’s Law in Vector Form
Coulomb’s law may be expressed also by its vector form. Let’s also focus on particle 1 and write the force acting on it in terms of a unit vector that points along a radial axis extending through the two particles, radially away from particle 2. As with other unit vectors, has a magnitude of exactly 1 and no unit. With these decisions, we write the electrostatic force as:
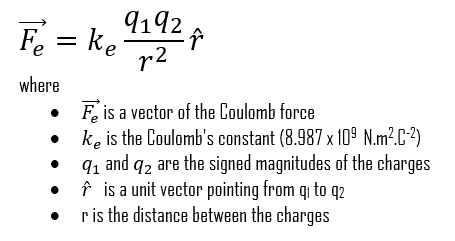
where r is the distance between the particles and k is a positive constant called the Coulomb constant.
Frequently asked questions
Coulomb’s Law has a many applications to modern life, from Xerox machines, laser printers, electrostatic air cleansing to powder coating.
Coulomb’s law can be used to calculate the force between charged particles (e.g., two protons) or between two charged objects. The magnitude of the electric force F is directly proportional to the amount of one electric charge, q1, multiplied by the other, q2, and inversely proportional to the square of the distance between the particles.

