30-second summary
Battery Discharging
Battery discharging occurs when the circuit is closed; the stronger attraction for the electrons by the cathode (e.g., LiCoO2 in lithium-ion batteries) will pull the electrons from the anode (e.g., lithium-graphite) through the wire in the circuit to the cathode electrode. During this process, each battery loses its voltage.
This battery chemical reaction, this flow of electrons through the wire, is electricity.
C-rate is used to express how fast a battery is discharged or charged relative to its maximum capacity. It has units h−1.
The coulometric capacity of a battery is the total Amp-hours available when the battery is discharged at a certain discharge current from 100% SOC to the cut-off voltage.
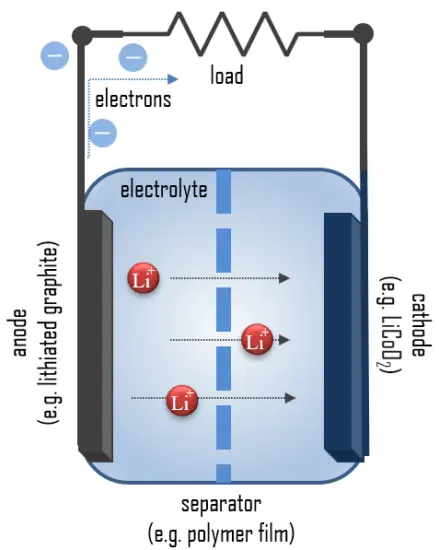
An electric battery is essentially a source of DC electrical energy. It converts stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. A typical battery consists of one or more voltaic cells.
The fundamental principle in an electrochemical cell is spontaneous redox reactions in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated.
Chemistry of Battery Discharging
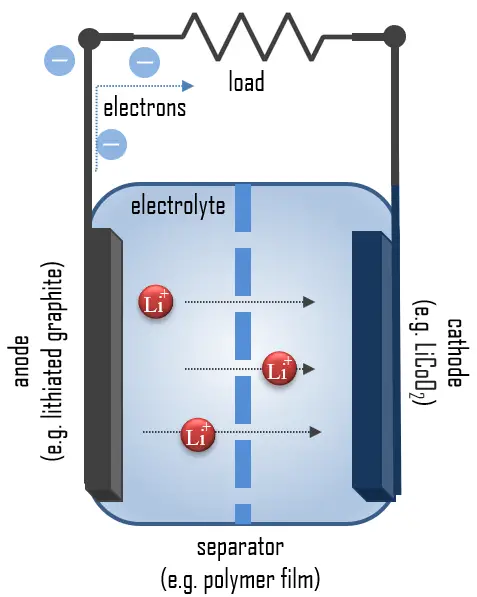
In simple terms, each battery is designed to keep the cathode and anode separated to prevent a reaction. The stored electrons will only flow when the circuit is closed. This happens when the battery is placed in a device, and the device is turned on.
When the circuit is closed, the stronger attraction for the electrons by the cathode (e.g., LiCoO2 in lithium-ion batteries) will pull the electrons from the anode (e.g., lithium-graphite) through the wire in the circuit to the cathode electrode. This battery chemical reaction, this flow of electrons through the wire, is electricity.
If we go into detail, batteries convert chemical energy directly to electrical energy. For example, chemical energy can be stored in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals. Lithium metal is the lightest metal and possesses a high specific capacity (3.86 Ah/g) and an extremely low electrode potential (−3.04 V vs. standard hydrogen electrode). Therefore lithium is an ideal anode material for high-voltage and high-energy batteries.
During discharge, lithium is oxidized from Li to Li+ (0 to +1 oxidation state) in the lithium-graphite anode through the following reaction:
C6Li → 6C(graphite) + Li+ + e–
These lithium ions migrate through the electrolyte medium to the cathode, where they are incorporated into lithium cobalt oxide through the following reaction, which reduces cobalt from a +4 to a +3 oxidation state:
CoO2 (s) + Li+ + e– → LiCoO2 (s)
Here is the full reaction (left to right = discharging, right to left = charging):
C6Li + CoO2 ⇄ C6 + LiCoO2
These reactions can be run in reverse to recharge the cell. In this case, the lithium ions leave the lithium cobalt oxide cathode and migrate back to the anode, where they are reduced back to neutral lithium and reincorporated into the graphite network.
C-rate of Battery
C-rate is used to express how fast a battery is discharged or charged relative to its maximum capacity. It has units h−1. The energy contained in a battery can be discharged at different rates, which means that the higher the discharge current is, the shorter the working time of the battery. In the same way, the lower the discharge current, the longer the time to discharge it completely will be needed. High C-rates generate more heat and cause the temperature of the cell to rise invoking the high-temperature degradation mechanisms. C-rate reduces the usable life and capacity of a battery. Manufacturers often publish datasheets with graphs showing capacity versus C-rate curves. A 1C rate means that the discharge current will discharge the entire battery in 1 hour. For a battery with a capacity of 100 Amp-hrs, this equates to a discharge current of 100 Amps. Standards for rechargeable batteries generally rate the capacity and charge cycles over a 4-hour (0.25C), 8 hour (0.125C) or longer discharge time.
- Alkaline battery. To obtain a reasonably good capacity reading, manufacturers commonly rate alkaline and lead acid batteries at a very low 0.05C, or a 20-hour discharge.
- According to Energizer, for NiMH batteries, the normal discharge rate is 0.2C. The battery nominal capacity is measured at this rate. At a higher discharge rate, the actual capacity of the battery will be much less than the nominal. The NiMH battery could be discharged at a maximum 3C rate.
- Most li-ion batteries can only withstand a maximum temperature of 60°C and are recommended to be charged at a maximum of 45°C under a 0.5C charge rate. C rating for a 18650 battery is usually 1C, this means that we can consume a maximum of 2.85A from the battery.
To avoid danger or premature capacity degradation, it is necessary to follow the manufacturer’s recommendations.
Capacity of Batteries
The coulometric capacity of a battery is the total Amp-hours available when the battery is discharged at a certain discharge current from 100% SOC to the cut-off voltage. It is determined by the amount of active material contained and represents the maximum amount of electrochemical energy that can be extracted from the battery.
Different battery chemistry and size creates different cell voltage and capacity.
- 0.2 Ah. CR2032 batteries use lithium manganese oxide technology to deliver a nominal voltage of 3V with a capacity of 210mAh. These coin cells have a diameter of 20mm and a thickness of only 3.2mm.
- 2 Ah – A typical alkaline or NiMH battery in the standard “AA” size has about 2000 to 3000 mAh (or 2 to 3 Ah).
- 3 Ah – Lithium Iron Disulfide (Li-FeS2) AA batteries are non-rechargeable batteries, featuring a nominal voltage of 1.5 volts and a typical capacity of 2700-3300 mAh, with some models having capacity up to 3500-3600 mAh.
- 3 Ah – Almost all lithium-ion batteries work at 3.8 volts. Lithium-ion 18650 batteries generally have capacity ratings from 2,300 to 3,600 mAh.
- 70 Ah – A common voltage for automobile batteries is 12 volts (DC). But this battery consists of six 2V lead cells. An average automotive battery might have a capacity of about 70 Ah, specified at a current of 3.5 A. This means that the amount of time this battery could continuously supply a current of 3.5 A to a load would be 20 hours (70 Ah / 3.5 A).
Self-discharge of Batteries
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied. The rate of side reactions is reduced for batteries stored at lower temperatures, although some can be damaged by freezing.
- Old rechargeable batteries self-discharge more rapidly than disposable alkaline batteries, especially nickel-based batteries; a freshly charged nickel-cadmium (NiCd) battery loses 10% of its charge in the first 24 hours and thereafter discharges at a rate of about 10% a month.
- The self-discharge of NIMH battery is 5–20% on the first day and stabilizes around 0.5–4% per day at room temperature. But at 45 °C it is approximately three times as high.
- A lead acid battery left in storage at moderate temperatures has an estimated self-discharge rate of 5% per month. This rate increases as temperatures rise and the risk of sulfation increases.
- Li-ion rechargeable batteries have a self-discharge rate typically stated by manufacturers to be 1.5–2% per month. The rate increases with temperature and state of charge.
- Primary batteries typically lose 2 to 5 percent of their original charge per year when stored at room temperature (20–30 °C).
- For the lithium-metal primary battery, such as the CR2032, the self-discharge rate is around 1% a year; this means a battery that is in storage.
A lower temperature reduces the rate of self-discharge, but manufacturers usually do not recommend such long-term storage.
Other Characteristics
To compare and understand the capability of each battery, some important parameters are characteristic of each battery, also within a type of battery. These parameters are a reference when a battery is needed, and specific qualities are required since batteries are used in all types of devices and for infinite purposes.
Cell Voltage
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
Cut-off Voltage
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
Capacity
The coulometric capacity is the total Amp-hours available when the battery is discharged at a certain discharge current from 100% SOC to the cut-off voltage.
C-rate of Battery
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
Self-discharge
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied.
Degradation
Some degradation of rechargeable batteries occurs on each charge–discharge cycle. Degradation usually occurs because electrolyte migrates away from the electrodes or because active material detaches from the electrodes.
Depth of Discharge
Depth of discharge is a measure of how much energy has been withdrawn from a battery and is expressed as a percentage of full capacity. For example, a 100 Ah battery from which 40 Ah has been withdrawn has undergone a 40% depth of discharge (DOD).
State of Charge
The state of charge refers to the amount of charge in a battery relative to its predefined “full” and “empty” states i.e. the amount of charge in Amp-hours left in the battery.









