30-second summary
Battery Cathode
The cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction. In the case of lithium batteries, cathode materials are generally constructed from LiCoO2 or LiMn2O4. For the cathode, it is important to hold a large amount of lithium without significant change in structure, have a good chemical and electrochemical stability with electrolyte, be a good electrical conductor and diffuser of lithium ions, and be of low cost.
See also: All about batteries
Composition of Battery
Batteries are made of an extensive range of materials resulting in different capabilities and behaviors in the functionality of the battery. The most common ones are lead, nickel, zinc, and lithium, each of them with different outputs and specific for some different purposes depending on the requirements. Many types of electrochemical cells have been produced, with varying chemical processes and designs, including galvanic cells, electrolytic cells, fuel cells, flow cells, and voltaic piles.
- A wet cell battery has a liquid electrolyte. Other names are flooded cell since the liquid covers all internal parts, or vented cell since gases produced during operation can escape to the air. Wet cells were a precursor to dry cells and are commonly used as a learning tool for electrochemistry.
- A dry cell uses a paste electrolyte with only enough moisture to allow current to flow. Unlike a wet cell, a dry cell can operate in any orientation without spilling, as it contains no free liquid, making it suitable for portable equipment.
The chemical and material composition of batteries determines their size, format, and overall performance. Therefore, each battery has a different composition. However, most batteries have some common components, although their material composition may vary.
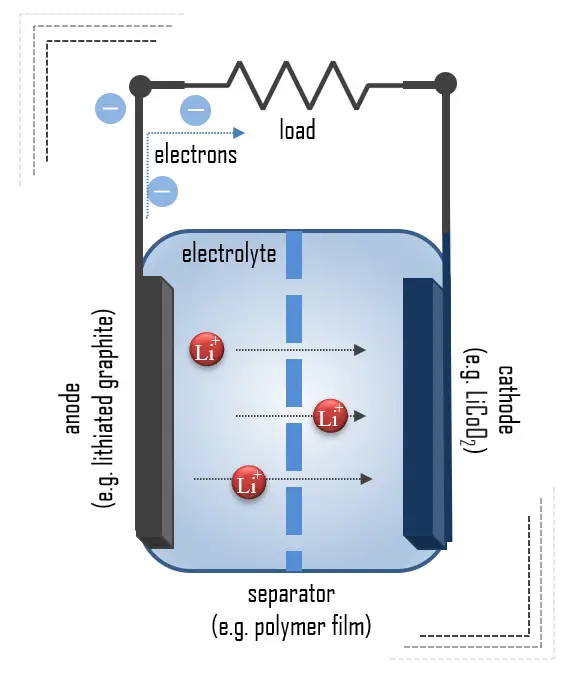
- Cathode. The cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction. In the case of lithium batteries, cathode materials are generally constructed from LiCoO2 or LiMn2O4. For the cathode, it is important to hold a large amount of lithium without significant change in structure, have a good chemical and electrochemical stability with electrolyte, be a good electrical conductor and diffuser of lithium ions, and be of low cost.
- Anode. The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during an electrochemical reaction. One of the most common anode materials used today is lithiated graphite, LixC6, which is composed of graphite sheets intercalated with lithium. New materials such as those based on Silicon and other elemental blends are being researched. Lithiated graphite has a unit cell with an HCP structure.
- Electrolyte. An electrolyte is a medium containing ions that are electrically conducting through the movement of those ions but not conducting electrons. The battery electrolyte is a solution inside batteries. Depending on the type of battery, it can be a liquid or paste-like substance. The choice of electrolyte in all batteries is critical for performance as well as safety. In lithium-ion batteries, the electrolyte is typically a lithium salt dissolved in organic solvents. A good electrolyte must have low reactivity with other cell components, high ionic conductivity, low toxicity, a large window of electrochemical voltage stability (0-5V), and be thermally stable. Aqueous potassium hydroxide is employed as the electrolyte in alkaline batteries based on nickel-cadmium, nickel-hydrogen, and manganese dioxide-zinc.
- Separator. A separator is a permeable membrane placed between a battery’s anode and cathode. The main function of a separator is to keep the two electrodes apart to prevent electrical short circuits while also allowing the transport of ionic charge carriers that are needed to close the circuit during the passage of current in an electrochemical cell. Commercially available liquid electrolyte cells use microporous polyolefin materials, such as polyethylene (PE) or polypropylene (PP). Separators in Li-ions have to be electrochemically and chemically stable relative to the electrolyte and electrode materials. Functional separators that use MOF-coated membranes to perform the dual functions of the electrolyte and separator are being developed to support the design of high-performance Li-metal batteries for high-energy systems in electric vehicles and electric aircraft.
- Current Collectors. Current collectors are the battery component responsible for transferring the flow of electrons from the electrodes to an external circuit. Several types of current collectors are available: mesh, foam, and foil. To minimize overall size and improve the volumetric capacity of cells, metallic foils which are thin and light are preferred. Current collectors are an electrochemically inactive volume in the cell but form the substrate that the electrochemically active materials are applied. Active materials are applied onto the thin current collectors with a conducting agent and an adhesive binder. Hence, current collectors should possess high electrical conductivity to reduce cell resistance as well as chemical stability in contact with liquid electrolyte over the operating voltage window of electrodes.
The alkaline battery consists of five parts:
- Inner current collector (pin)
- Anode. The active material in the anode is Zn. With a standard electrode potential (SEP) of −0.76 volts, zinc is used as an anode material for batteries. (More reactive lithium (SEP −3.04 V) is used for anodes in lithium batteries ).
- Separator. A separator is a permeable membrane placed between a battery’s anode and cathode. For example, non-woven, fibrous fabric that separates the electrodes.
- Cathode. The active material of the cathode is manganese dioxide. The principal use for MnO2 is for dry-cell batteries, such as the alkaline battery and the zinc-carbon battery.
- Electrolyte. Aqueous potassium hydroxide is employed as the electrolyte in alkaline batteries.
- Outer current collector (can)



