30-second summary
Dielectric Constant
The dielectric constant of an insulator measures the ability of the insulator or dielectric to store electric energy in an electrical field.
The dielectric constant, which is denoted by κ (kappa), is the same quantity as the relative permittivity denoted by εr .
The dielectric constant is still used but deprecated by standards organizations in engineering.
See also: Capacitance
See also: Electric polarisation
See also: Permittivity
Dielectric Constant
The dielectric constant of an insulator measures the ability of the insulator or dielectric to store electric energy in an electrical field. The dielectric constant, which is denoted by κ (kappa), is the same quantity as the relative permittivity denoted by εr . The dielectric constant is still used but deprecated by standards organizations in engineering.
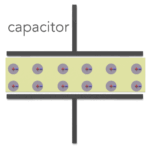
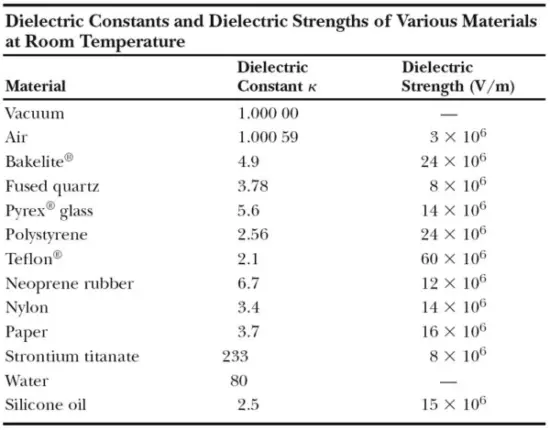
The dielectric constant of a vacuum is unity by definition. Because air is mostly empty space, its measured dielectric constant is only slightly greater than unity. Even common paper can significantly increase the capacitance of a capacitor, and some materials, such as strontium titanate, can increase the capacitance by more than two orders of magnitude.
In a region completely filled by a dielectric material of dielectric constant κe, all electrostatic equations containing the permittivity constant ε0 are to be modified by replacing ε0 with κeε0 .
The greater the dielectric constant, the greater the amount of charge that can be held. The capacitance of a capacitor is increased by a factor of the dielectric constant when the gap between the plates is completely filled with a dielectric.
C = εrC0 = κeC0
where C0 is the capacitance between the plates with no dielectric.
Dielectrics
In general, a dielectric is an electrical insulator with high permittivity, which means with high polarizability. When a dielectric material is placed in an electric field, electric charges do not flow through the material as they do in an electrical conductor because they have no loosely bound or free electrons that may drift through the material, but instead, they shift only slightly, from their average equilibrium positions, causing dielectric polarization.
Dielectric materials can be solid, liquid, or gaseous, and their electrical properties depend on factors such as their chemical composition, temperature, and frequency of the electrical field. Common examples of solid dielectric materials include ceramics, glass, plastics, and certain types of crystals, such as quartz.
The electrical properties of dielectric materials are characterized by their permittivity, which is a measure of the material’s ability to store electrical energy in an electric field. Dielectric materials with a high permittivity can store more electrical energy than those with a low permittivity. The permittivity of a material also determines its capacitance, which is the ability of a material to store electrical energy in a capacitor.