30-second summary
Peltier Effect – Peltier Cooling
The Peltier effect is the analog to the Seebeck effect. The Peltier effect is the presence of heating or cooling at an electrified junction of two different conductors and is named after French physicist Jean Charles Athanase Peltier, who discovered it in 1834.
Instead of generating a potential difference across C and D with junctions J1 and J2 held at different temperatures, the Peltier effect uses an electromotive source to drive a current, heating one junction and cooling the other.
See more: Thermoelectric effect
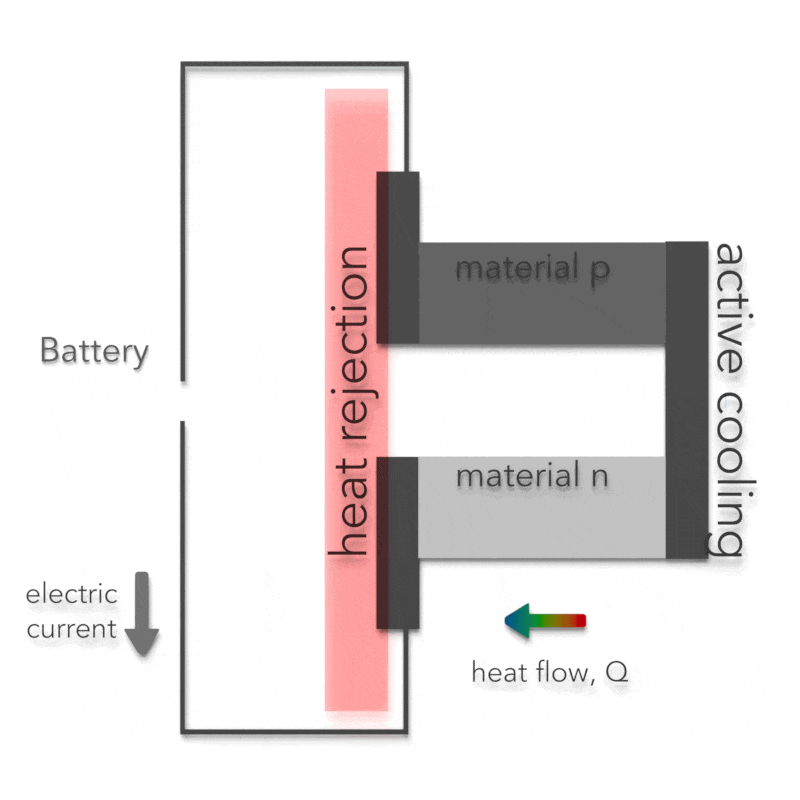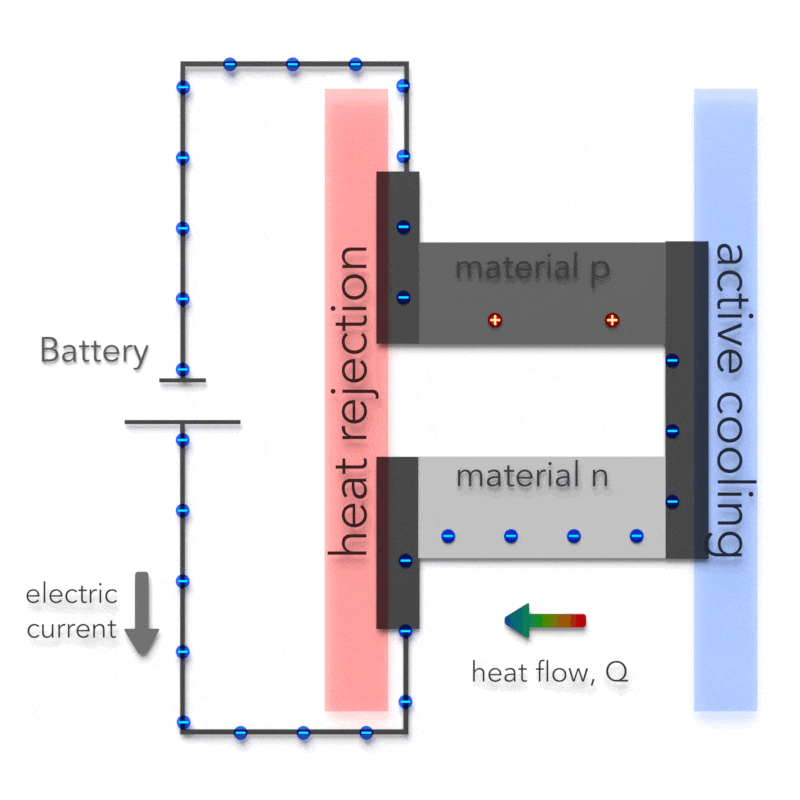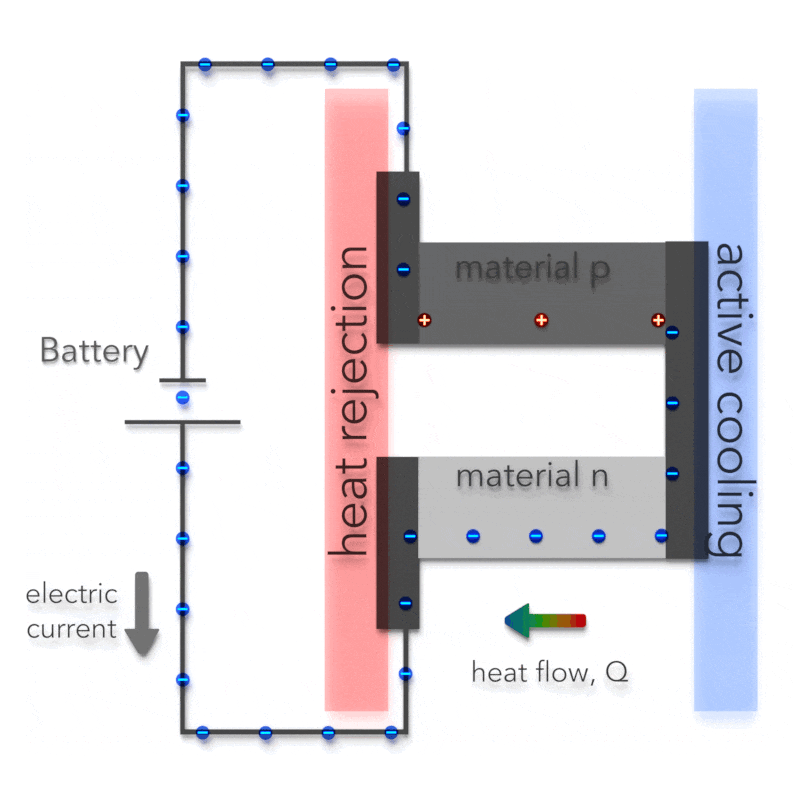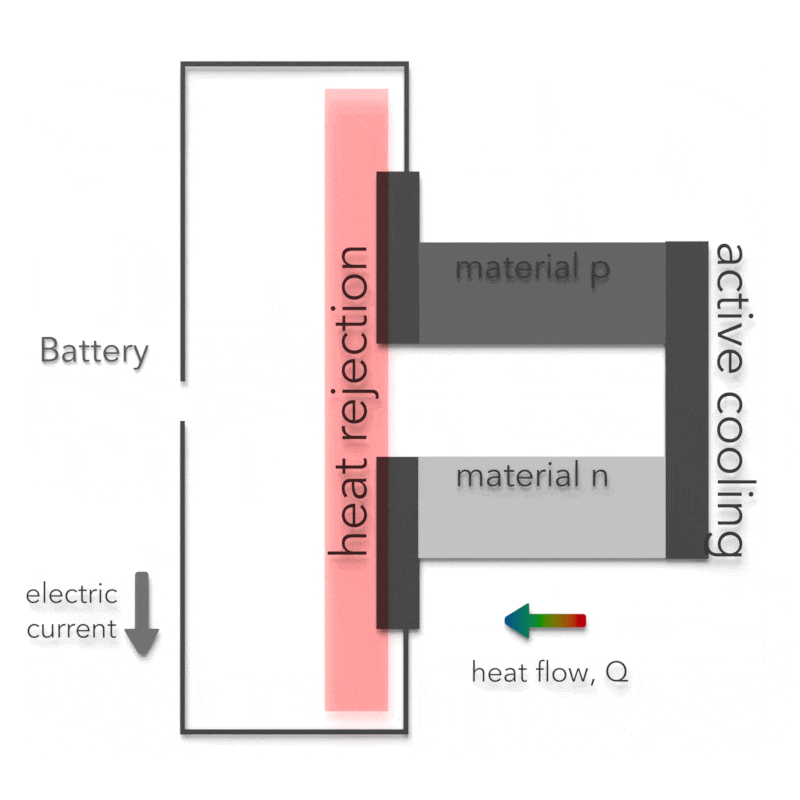
Peltier Effect – Peltier Cooling
The Peltier effect is the analog to the Seebeck effect. The Peltier effect is the presence of heating or cooling at an electrified junction of two different conductors and is named after French physicist Jean Charles Athanase Peltier, who discovered it in 1834. Instead of generating a potential difference across C and D with junctions J1 and J2 held at different temperatures, the Peltier effect uses an electromotive source to drive a current, heating one junction and cooling the other. The effect can be quantitatively described by the Peltier coefficient. The Peltier coefficient (π) is determined by the ratio of the current, (I) to the rate of heating (q): π = I/q. It represents how much heat is carried per unit charge. The sign of π is determined by which junction is heated and which is cooled. A typical Peltier heat pump involves multiple junctions in series, through which a current is driven. Some of the junctions lose heat due to the Peltier effect, while others gain heat. Thermoelectric heat pumps exploit this phenomenon, as do thermoelectric cooling devices found in refrigerators.
Thomson effect. In different materials, the Seebeck coefficient is not constant in temperature, and so a spatial gradient in temperature can result in a gradient in the Seebeck coefficient. If a current is driven through this gradient, then a continuous version of the Peltier effect will occur. The Thomson effect describes the resulting electric current that develops in a single conductor when a small temperature gradient is applied. This relationship is described by the equation; q = βIΔT, where q is the rate of heating, I is an electric current, ΔT is the change in temperature, and β is the Thomson coefficient. Lord Kelvin, tied all three of the thermoelectric coefficients together in the Kelvin relationships. These equations describe how the Seebeck, Peltier, and Thomson coefficients interrelate.
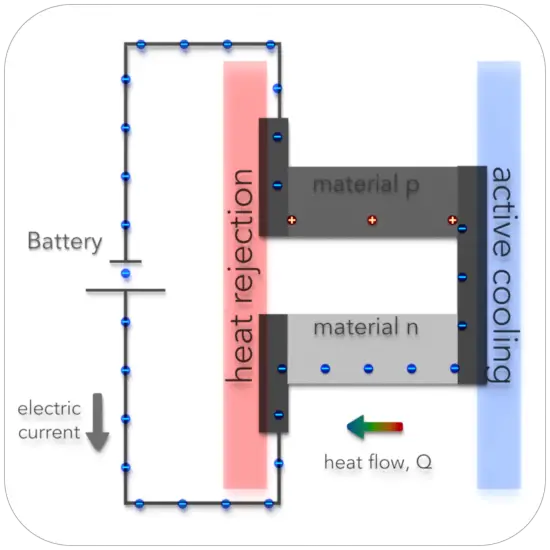
The Peltier effect can be considered as the back-action counterpart to the Seebeck effect: if a simple thermoelectric circuit is closed, then the Seebeck effect will drive a current, which in turn (by the Peltier effect) will always transfer heat from the hot to the cold junction. The close relationship between Peltier and Seebeck effects can be seen in the direct connection between their coefficients:
The second Thomson relation:
Π = TS
where Π is the Peltier coefficient, T is the absolute temperature, and S is the Seebeck coefficient.
Thermoelectric Materials
Thermoelectric materials convert thermal energy into electrical energy by a process known as thermoelectric conversion. The heat source giving rise to the temperature difference can be combustion engines, sunlight, chemical reactions, or nuclear decay.
These materials must have both high electrical conductivity (σ) and low thermal conductivity (κ) to be good thermoelectric materials. Having low thermal conductivity ensures that when one side is made hot, the other side stays cold, which helps to generate a large voltage while in a temperature gradient.
Three materials are commonly used for thermoelectric generators. For many years, the main three semiconductors known to have both low thermal conductivity and high power factor are bismuth (Bi2Te3) telluride, lead telluride (PbTe), and Silicon germanium (SiGe). Which material is used depends on the characteristics of the heat source, cold sink, and the design of the thermoelectric generator. Today, the thermal conductivity of semiconductors can be lowered without affecting their high electrical properties using nanotechnology. This can be achieved by creating nanoscale features such as particles, wires, or interfaces in bulk semiconductor materials.

