30-second summary
Conservation of Electric Charge
An electric charge is a physical quantity and property of matter that causes it to experience a force when placed in an electromagnetic field.
The law of conservation of electric charge states that:
The algebraic sum of all the electric charges in any closed system is constant.
The second important principle is:
The magnitude of the charge of the electron or proton is a natural unit of charge.
Law of Conservation of Electric Charge
In physics, there are two very important principles concerning the electric charge.
First is the law of conservation of electric charge. This law states that:
The algebraic sum of all the electric charges in any closed system is constant.
The only way to change the net charge of a system is to bring in charge from elsewhere or remove a charge from the system. The charge can be created and destroyed, but only in positive-negative pairs.
Conservation of charge is thought to be a universal conservation law. No experimental evidence for any violation of this principle has ever been observed. In particle physics, charge conservation means that in elementary particle reactions that create charged particles, equal numbers of positive and negative particles are always created, keeping the net amount of charge unchanged. Even in high-energy interactions in which particles are created and destroyed, such as the creation of positron-electron pairs, the total charge of any closed system is exactly constant.
The second important principle is:
The magnitude of the charge of the electron or proton is a natural unit of charge.
We say that charge is quantized. That is, every observable amount of electric charge is always an integer multiple of this basic unit. This unit is called the elementary charge, e, approximately equal to 1.602×10−19 coulombs (except for particles called quarks, which have charges that are integer multiples of 1⁄3e).
Frequently asked questions
An atom consists of a positively charged nucleus surrounded by negatively charged electrons so that the atom as a whole is electrically neutral. The atomic nucleus consists of positively charged protons and neutral neutrons.
The coulomb (symbol: C) is the International System of Units (SI) unit of electric charge. The coulomb was defined as the quantity of electricity transported in one second by a current of one ampere: 1 C = 1 A × 1 s
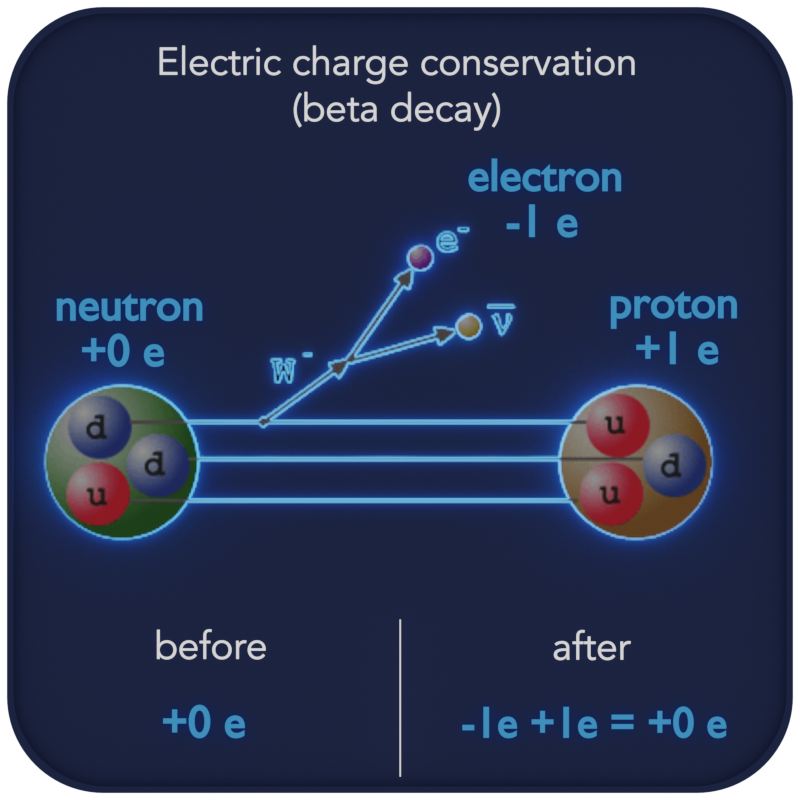
This concept is important for all nuclear reactions—alpha decay, beta decay, gamma decay, etc. — because it allows scientists to predict the composition of the final product in the reaction. In these reactions, a nucleus transforms into a different type of nucleus or nuclei, while the charge is conserved.