30-second summary
Electric Charge
An electric charge is a physical quantity and property of matter that causes it to experience a force when placed in an electromagnetic field.
There are two types of electric charge:
positive, transmitted by protons
negative, transmitted by electrons
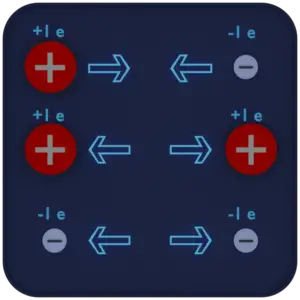
If the total charge is zero, it is said to be neutral. The same charges are repelled and the opposite charges are attracted.
The electrons in an atom are attracted to the protons in the nucleus by the electromagnetic force.
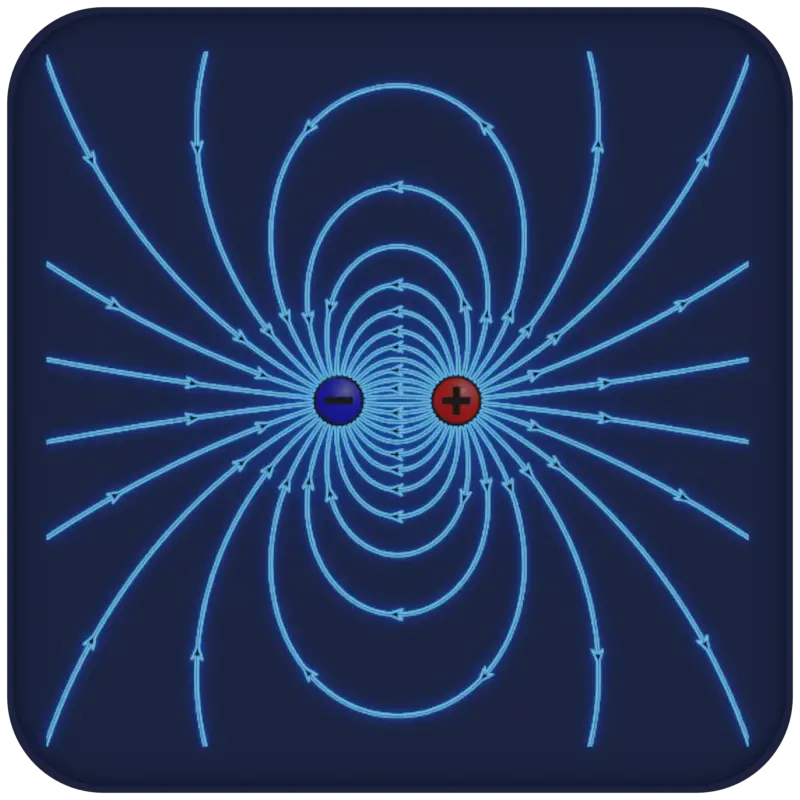
About Electric Charge
An electric charge is a physical quantity and property of matter that causes it to experience a force when placed in an electromagnetic field. There are two types of electric charge: positive, transmitted by protons, and negative, transmitted by electrons. If the total charge is zero, it is said to be neutral. The same charges are repelled and the opposite charges are attracted. These facts are known as the First Law of Electrostatics and are sometimes referred to as the law of electrical charges.
Elementary Charge
The most fundamental unit of charge is the magnitude of the charge of an electron or a proton, which is denoted by e. The most precise value available is:
e = 1.602176487 x 10-19C
One coulomb represents the negative of the total charge of about 6 x 1018 electrons.
We rarely encounter charges as large as a coulomb. Charges produced by rubbing ordinary objects (such as a comb or plastic ruler) are typically around a microcoulomb (?C = 10-6 C) or less. In an ordinary 100 W lightbulb, for example, about 1019 elementary charges enter the bulb every second and just as many leave.
The proton has charge + e and electron – e. The charge is quantized; it comes in integer multiples of individual small units called the elementary charge, e, which is the smallest charge which can exist freely (particles called quarks have smaller charges, multiples of ⅓ e, but they are only found in combination, and always combine to form particles with integer charge). The proton has a quark composition of uud, and so its charge quantum number is:
q(uud) = 2/3 + 2/3 + (-1/3) = +1e.
The neutron has a quark composition of udd, and its charge quantum number is therefore:
q(udd) = 2/3 + (-1/3) + (-1/3) = 0
Since the neutron has no net electric charge, it is not affected by electric forces, but the neutron does have a slight distribution of electric charge within it. This is caused by by its internal quark structure. This results in non-zero magnetic moment (dipole moment) of the neutron. Therefore the neutron interacts also via electromagnetic interaction, but much weaker than the proton.
Law of Conservation of Electric Charge
In physics, there are two very important principles concerning the electric charge.
First is the law of conservation of electric charge. This law states that:
The algebraic sum of all the electric charges in any closed system is constant.
The only way to change the net charge of a system is to bring in charge from elsewhere or remove a charge from the system. The charge can be created and destroyed, but only in positive-negative pairs.
Conservation of charge is thought to be a universal conservation law. No experimental evidence for any violation of this principle has ever been observed. In particle physics, charge conservation means that in elementary particle reactions that create charged particles, equal numbers of positive and negative particles are always created, keeping the net amount of charge unchanged. Even in high-energy interactions in which particles are created and destroyed, such as the creation of positron-electron pairs, the total charge of any closed system is exactly constant.
The second important principle is:
The magnitude of the charge of the electron or proton is a natural unit of charge.
We say that charge is quantized. That is, every observable amount of electric charge is always an integer multiple of this basic unit. This unit is called the elementary charge, e, approximately equal to 1.602×10−19 coulombs (except for particles called quarks, which have charges that are integer multiples of 1⁄3e).
Electric Charge of Antiparticles
Theoretically, a particle and its anti-particle (for example, a proton and an antiproton) have the same mass, but opposite electric charge, and other differences in quantum numbers. For example, for every quark there is a corresponding type of antiparticle. The antiquarks have the same mass, mean lifetime, and spin as their respective quarks, but the electric charge and other charges have the opposite sign. That means a proton has positive charge while an antiproton has negative charge and therefore they attract each other. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles can be totally annihilated, producing gamma ray photons.
Example: Electric Charge
Noteworthy, in four liters of water, there is about 2.1 x 108C of total electron charge. Thus, if we place two bottles a meter apart, the electrons in one of the bottles repel those in the other bottle with a force of 4.1 x 1026N. This tremendous force is comparable with the force that the planet Earth would weigh if weighed on another Earth. Ut, as was written, there are also positive (protons) and these charges tend to cancel each other out.
Frequently asked questions
The atom’s chemical properties are determined by the number of protons, in fact, by the number and arrangement of electrons. The configuration of these electrons follows the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor determining its chemical bonding behavior.
An atom consists of a positively charged nucleus surrounded by negatively charged electrons so that the atom as a whole is electrically neutral. The atomic nucleus consists of positively charged protons and neutral neutrons.
The electrons in an atom are attracted to the protons in the nucleus by the electromagnetic force. This force binds the electrons inside an electrostatic potential well surrounding the smaller nucleus, which means that an external source of energy is needed for the electron to escape.
The coulomb (symbol: C) is the International System of Units (SI) unit of electric charge. The coulomb was defined as the quantity of electricity transported in one second by a current of one ampere: 1 C = 1 A × 1 s

