Semiconductors
In general, semiconductors are inorganic or organic materials that can control their conduction depending on chemical structure, temperature, illumination, and the presence of dopants. The name semiconductor comes from the fact that these materials have electrical conductivity between a metal, like copper, gold, etc., and an insulator, like glass. They have an energy gap of less than 4eV (about 1eV). In solid-state physics, this energy gap or band gap is an energy range between the valence band and conduction band where electron states are forbidden. In contrast to conductors, semiconductors’ electrons must obtain energy (e.g., from ionizing radiation) to cross the band gap and reach the conduction band. The properties of semiconductors are determined by the energy gap between valence and conduction bands.
Types of Semiconductors
Semiconductors can be classified into two basic types based on their electronic properties:
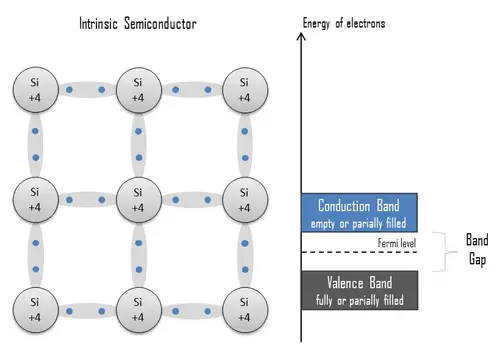
- Intrinsic Semiconductors: These are pure semiconductors that are made up of a single element (e.g., Silicon, Germanium) and have no intentional doping with impurities. Intrinsic semiconductors have a specific number of electrons in their valence band and conduction band. They conduct electricity when they are heated, and some electrons gain sufficient energy to break free from their bonds and become free electrons in the conduction band.
- Extrinsic Semiconductors: These are impure semiconductors that are intentionally doped with impurities to change their electronic properties. Extrinsic semiconductors can be further classified into two types:
- p-type semiconductors: In p-type semiconductors, impurity atoms such as boron are introduced into the semiconductor material. These impurities have fewer valence electrons than the semiconductor material, which results in “holes” (absence of electrons) being created in the valence band. These holes can conduct current like positive charge carriers, which gives the material its p-type designation.
- n-type semiconductors: In n-type semiconductors, impurity atoms such as phosphorus are introduced into the semiconductor material. These impurities have more valence electrons than the semiconductor material, which creates excess electrons in the conduction band. These excess electrons can conduct current like negative charge carriers, which gives the material its n-type designation.
Here is a table with 3 intrinsic semiconductors and 2 p-type and n-type semiconductors, along with 4 key properties:
| Semiconductor | Type | Band Gap (eV) | Electron Mobility (cm²/Vs) | Hole Mobility (cm²/Vs) | Thermal Conductivity (W/mK) |
|---|---|---|---|---|---|
| Silicon (Si) | Intrinsic | 1.12 | 1500 | 450 | 150 |
| Germanium (Ge) | Intrinsic | 0.67 | 3900 | 1900 | 60 |
| Gallium Arsenide (GaAs) | Intrinsic | 1.43 | 8500 | 400 | 46 |
| Boron-doped Silicon (p-Si) | p-type | 1.12 | 1500 | 1800 | 150 |
| Phosphorus-doped Silicon (n-Si) | n-type | 1.12 | 1500 | 4500 | 150 |
| Aluminum-doped Gallium Arsenide (p-GaAs) | p-type | 1.43 | 8500 | 200 | 46 |
| Silicon-doped Gallium Arsenide (n-GaAs) | n-type | 1.43 | 8500 | 800 | 46 |
Extrinsic Semiconductors – Doped Semiconductors
An extrinsic semiconductor, or doped semiconductor, is a semiconductor that was intentionally doped to modulate its electrical, optical, and structural properties. In the case of semiconductor detectors of ionizing radiation, doping is the intentional introduction of impurities into an intrinsic semiconductor for the purpose of changes in their electrical properties. Therefore, intrinsic semiconductors are also known as pure semiconductors or i-type semiconductors.
The addition of a small percentage of foreign atoms in the regular crystal lattice of silicon or germanium produces dramatic changes in their electrical properties since these foreign atoms incorporated into the crystal structure of the semiconductor provide free charge carriers (electrons or electron holes) in the semiconductor. In an extrinsic semiconductor, these foreign dopant atoms in the crystal lattice mainly provide the charge carriers that carry electric current through the crystal. Two types of dopant atoms generally result in two types of extrinsic semiconductors. These dopants that produce the desired controlled changes are classified as either electron acceptors or donors, and the corresponding doped semiconductors are known as:
- n-type Semiconductors.
- p-type Semiconductors.
Extrinsic semiconductors are components of many common electrical devices, as well as many detectors of ionizing radiation. For these purposes, a semiconductor diode (devices that allow current in only one direction) usually consists of p-type and n-type semiconductors placed in a junction with one another.
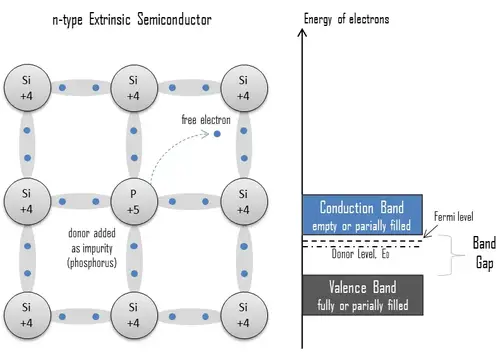
n-type Semiconductors
An extrinsic semiconductor doped with electron donor atoms is called an n-type semiconductor because most charge carriers in the crystal are negative electrons. Since silicon is a tetravalent element, the normal crystal structure contains 4 covalent bonds from four valence electrons. The most common dopants in silicon are group III and V elements. Group V elements (pentavalent) have five valence electrons, allowing them to act as donors. That means adding these pentavalent impurities such as arsenic, antimony, or phosphorus contributes to free electrons, greatly increasing the conductivity of the intrinsic semiconductor. For example, a silicon crystal doped with boron (group III) creates a p-type semiconductor, whereas a crystal doped with phosphorus (group V) results in an n-type semiconductor.
The conduction electrons are completely dominated by the number of donor electrons. Therefore:
The total number of conduction electrons is approximately equal to the number of donor sites, n≈ND.
The charge neutrality of semiconductor material is maintained because excited donor sites balance the conduction electrons. The net result is that the number of conduction electrons increases while the number of holes is reduced. The imbalance of the carrier concentration in the respective bands is expressed by the different absolute number of electrons and holes. Electrons are majority carriers, while holes are minority carriers in n-type material.
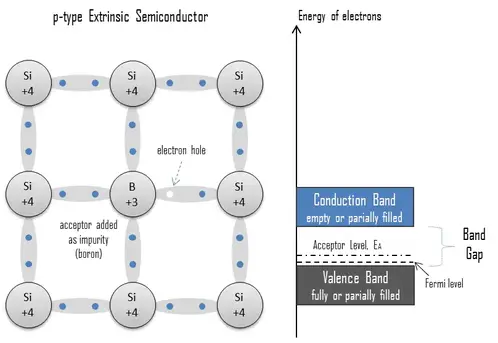
p-type Semiconductors
An extrinsic semiconductor doped with electron acceptor atoms is called a p-type semiconductor because most charge carriers in the crystal are electron holes (positive charge carriers). The pure semiconductor silicon is a tetravalent element, and the normal crystal structure contains 4 covalent bonds from four valence electrons. In silicon, the most common dopants are group III and group V elements. Group III elements (trivalent) all contain three valence electrons, causing them to function as acceptors when used to dope silicon. When an acceptor atom replaces a tetravalent silicon atom in the crystal, a vacant state (an electron-hole) is created. An electron-hole (often simply called a hole) is the lack of an electron at a position where one could exist in an atom or atomic lattice. It is one of the two charge carriers responsible for creating an electric current in semiconducting materials. These positively charged holes can move from atom to atom in semiconducting materials as electrons leave their positions. Adding trivalent impurities such as boron, aluminum, or gallium to an intrinsic semiconductor creates these positive electron holes in the structure. For example, a silicon crystal doped with boron (group III) creates a p-type semiconductor, whereas a crystal doped with phosphorus (group V) results in an n-type semiconductor.
The number of acceptor sites completely dominates the number of electron holes. Therefore:
The total number of holes is approximately equal to the number of donor sites, p ≈ NA.
The charge neutrality of this semiconductor material is also maintained. The net result is that the number of electron holes is increased while the number of conduction electrons is reduced. The imbalance of the carrier concentration in the respective bands is expressed by the different absolute number of electrons and holes. Electron holes are majority carriers, while electrons are minority carriers in p-type material.