Semiconductors
In general, semiconductors are inorganic or organic materials that can control their conduction depending on chemical structure, temperature, illumination, and the presence of dopants. The name semiconductor comes from the fact that these materials have electrical conductivity between a metal, like copper, gold, etc., and an insulator, like glass. They have an energy gap of less than 4eV (about 1eV). In solid-state physics, this energy gap or band gap is an energy range between the valence band and conduction band where electron states are forbidden. In contrast to conductors, semiconductors’ electrons must obtain energy (e.g., from ionizing radiation) to cross the band gap and reach the conduction band. The properties of semiconductors are determined by the energy gap between valence and conduction bands.
p-type Semiconductors
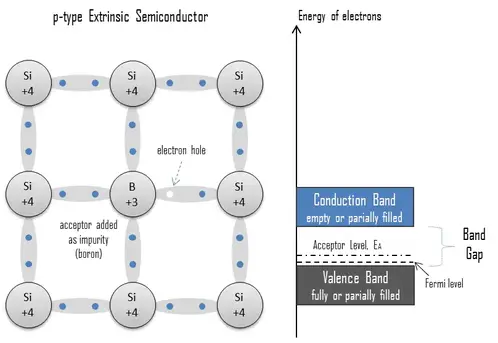
An extrinsic semiconductor doped with electron acceptor atoms is called a p-type semiconductor because most charge carriers in the crystal are electron holes (positive charge carriers). The pure semiconductor silicon is a tetravalent element, and the normal crystal structure contains 4 covalent bonds from four valence electrons. In silicon, the most common dopants are group III and group V elements. Group III elements (trivalent) all contain three valence electrons, causing them to function as acceptors when used to dope silicon. When an acceptor atom replaces a tetravalent silicon atom in the crystal, a vacant state (an electron-hole) is created. An electron-hole (often simply called a hole) is the lack of an electron at a position where one could exist in an atom or atomic lattice. It is one of the two charge carriers responsible for creating an electric current in semiconducting materials. These positively charged holes can move from atom to atom in semiconducting materials as electrons leave their positions. Adding trivalent impurities such as boron, aluminum, or gallium to an intrinsic semiconductor creates these positive electron holes in the structure. For example, a silicon crystal doped with boron (group III) creates a p-type semiconductor, whereas a crystal doped with phosphorus (group V) results in an n-type semiconductor.
The number of acceptor sites completely dominates the number of electron holes. Therefore:
The total number of holes is approximately equal to the number of donor sites, p ≈ NA.
The charge neutrality of this semiconductor material is also maintained. The net result is that the number of electron holes is increased while the number of conduction electrons is reduced. The imbalance of the carrier concentration in the respective bands is expressed by the different absolute number of electrons and holes. Electron holes are majority carriers, while electrons are minority carriers in p-type material.
Here is a table with 3 intrinsic semiconductors and 2 p-type and n-type semiconductors, along with 4 key properties:
| Semiconductor | Type | Band Gap (eV) | Electron Mobility (cm²/Vs) | Hole Mobility (cm²/Vs) | Thermal Conductivity (W/mK) |
|---|---|---|---|---|---|
| Silicon (Si) | Intrinsic | 1.12 | 1500 | 450 | 150 |
| Germanium (Ge) | Intrinsic | 0.67 | 3900 | 1900 | 60 |
| Gallium Arsenide (GaAs) | Intrinsic | 1.43 | 8500 | 400 | 46 |
| Boron-doped Silicon (p-Si) | p-type | 1.12 | 1500 | 1800 | 150 |
| Phosphorus-doped Silicon (n-Si) | n-type | 1.12 | 1500 | 4500 | 150 |
| Aluminum-doped Gallium Arsenide (p-GaAs) | p-type | 1.43 | 8500 | 200 | 46 |
| Silicon-doped Gallium Arsenide (n-GaAs) | n-type | 1.43 | 8500 | 800 | 46 |