30-second summary
Coulomb – Unit of Charge
The coulomb (symbol: C) is the International System of Units (SI) unit of electric charge. The coulomb was defined as the quantity of electricity transported in one second by a current of one ampere:
1 C = 1 A × 1 s
The 2019 redefinition of the ampere and other SI base units fixed the numerical value of the elementary charge when expressed in coulombs, and therefore fixed the value of the coulomb when expressed as a multiple of the fundamental charge.
Thus, one coulomb is the charge of approximately 6241509074460762607.776 elementary charges. It is impossible to realize exactly 1 C of charge, since the number of elementary charges is not an integer.

About Electric Charge
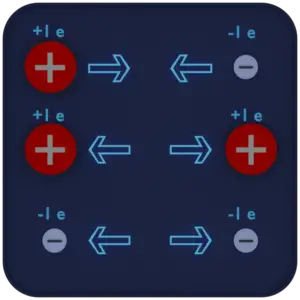
An electric charge is a physical quantity and property of matter that causes it to experience a force when placed in an electromagnetic field. There are two types of electric charge: positive, transmitted by protons, and negative, transmitted by electrons. If the total charge is zero, it is said to be neutral. The same charges are repelled and the opposite charges are attracted. These facts are known as the First Law of Electrostatics and are sometimes referred to as the law of electrical charges.
Elementary Charge
The most fundamental unit of charge is the magnitude of the charge of an electron or a proton, which is denoted by e. The most precise value available is:
e = 1.602176487 x 10-19C
One coulomb represents the negative of the total charge of about 6 x 1018 electrons.
We rarely encounter charges as large as a coulomb. Charges produced by rubbing ordinary objects (such as a comb or plastic ruler) are typically around a microcoulomb (?C = 10-6 C) or less.
Example: Electric Charge
Noteworthy, in four liters of water, there is about 2.1 x 108C of total electron charge. Thus, if we place two bottles a meter apart, the electrons in one of the bottles repel those in the other bottle with a force of 4.1 x 1026N. This tremendous force is comparable with the force that the planet Earth would weigh if weighed on another Earth. Ut, as was written, there are also positive (protons) and these charges tend to cancel each other out.
Frequently asked questions
The coulomb (symbol: C) is the International System of Units (SI) unit of electric charge. The coulomb was defined as the quantity of electricity transported in one second by a current of one ampere: 1 C = 1 A × 1 s
One coulomb is the charge of approximately 6241509074460762607.776 elementary charges. It is impossible to realize exactly 1 C of charge, since the number of elementary charges is not an integer.
A typical alkaline or NiMH battery in the standard “AA” size has about 2000 to 3000 mAh (or 2 to 3 Ah). With a cell voltage of 1.2 V to 1.5V. Thus, an AA battery can transport about 3 x 60 x 60 = 10800 Coulombs.

