Nickel Metal Hydride Battery
A nickel metal hydride battery, NiMH, is a rechargeable battery with a positive electrode made of nickel hydroxide and a negative electrode made of a metal hydride (a hydrogen-absorbing alloy). The NiMH battery was commercially introduced in 1989 and was mainly used as a power source in portable personal computers. Since then, the NiMH battery system has become very popular in electric hybrid vehicles and makes up 10% of the total market for rechargeable batteries. Compared to the NiCd battery, the NiMH provides 40 percent higher specific energy resulting in about two times higher capacity. NiMH batteries are also less affected by voltage depression, but the main advantage is the absence of toxic cadmium. The memory effect of NiMH batteries is much less than nickel-cadmium batteries.
Chemistry of Nickel Metal Hydride Battery – How it works
A typical NiMH cell contains:
- Cathode: a nickel(III) oxide-hydroxide positive electrode plate
- Anode: a metal hydride (a hydrogen-absorbing alloy)
- Separator
- Electrolyte: an alkaline electrolyte (potassium hydroxide).
The metal M in the negative electrode of a NiMH cell is an intermetallic compound. Many different compounds have been developed for this application. The research on materials for negative electrodes for the Ni/MH battery has involved several alloy families: AB5, AB2, and AB. In this notation, A represents a metallic element with a strong affinity to hydrogen (typically rare-earth element like lanthanum, cerium, neodymium, or praseodymium), whereas B is a metallic element with weak hydrogen affinity (typically transition metal like nickel, cobalt, manganese, or aluminum). Today the hydride-forming materials based on the AB5 intermetallics are the most versatile and commercially important family of reversible hydriding alloys. The optimal composition for electrode purposes has been found for an alloy with the following composition:
MmNi3.55Mn0.4Al0.3Co0.75
where Mm is a Mischmetal, which is an alloy of rare-earth elements. It is also called cerium mischmetal or rare-earth mischmetal. A typical composition includes approximately 55% cerium, 25% lanthanum, and 15~18% neodymium, with traces of other rare earth metals.
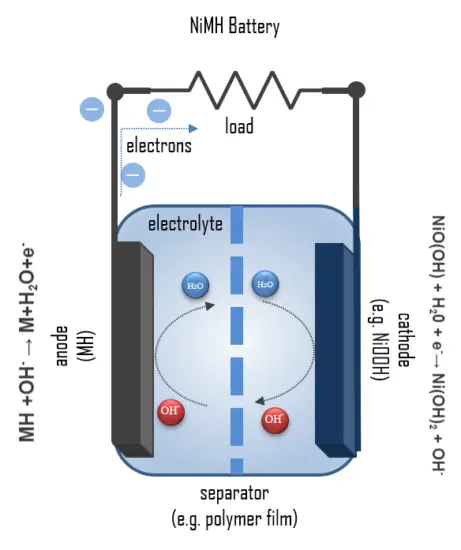
The layer structure of a NiMH round cell can be seen in the figure. An outer perforated foil serves as a carrier for the metal hydride powder, which forms the negative electrode. During discharge, the hydrogen bound in the metal hydride MH is oxidized to a proton, and metal of 0 oxidation state is formed:
MH +OH– → M+H2O+e–
The resulting protons react with the OH– hydroxide ions of the KOH solution to form water. The redox potential is approximately -0.83 V. The separator holds the electrolyte, a 20% KOH solution, and prevents direct contact with the positive electrode. The cathode consists of a sheet of nickel hydroxide and nickel oxide hydrate. Here, nickel of oxidation state +III is reduced to nickel of oxidation state +II in the reaction:
NiO(OH) + H20 + e–→ Ni(OH)2 + OH–
The overall reaction during discharge is:
NiO(OH) + MH → Ni(OH)2 + M
During this process, free electrons are bound so that this pole becomes the positive electrode. The redox voltage of the reduction is approximately 0.49 V. The total voltage of the redox reaction is thus E0 = 0.49V – ( – 0.83V) = 1.32V.
The specific energy of a NiMH cell is about 80 Wh/kg, which is almost as high as that of an alkaline cell and more than twice as high as that of a NiCd battery. NiMH batteries are sensitive to overcharging, overheating, incorrect polarity, and also to deep discharge.