Nickel-cadmium Battery
The nickel-cadmium battery (Ni-Cd battery) is a type of secondary battery using nickel oxide hydroxide Ni(O)(OH) as a cathode and metallic cadmium as an anode. The abbreviation Ni-Cd is derived from the chemical symbols of nickel (Ni) and cadmium (Cd).
The battery has low internal impedance resulting in high power capabilities but lower energy storage capacity compared to other battery systems. It has long cycle life and the capability of rapid recharge but may suffer from voltage depression or memory effect, meaning that the maximum charge voltage will decrease and hence the energy capacity if continuously discharged shallowly. The greatest disadvantage is the content of cadmium. Unfortunately, cadmium is extremely toxic; therefore, the Ni-Cd will not be an alternative for a modern battery system.
Nowadays, the applications of nickel-cadmium batteries are in small-size portable devices such as power tools, toys, emergency lighting, medical instrumentation, or industrial portable products. It is used in small-size products because their cost for low-power applications is inexpensive but three to four times more expensive than lead-acid batteries for the same capacity.
Chemistry of Nickel-cadmium Battery – How it works
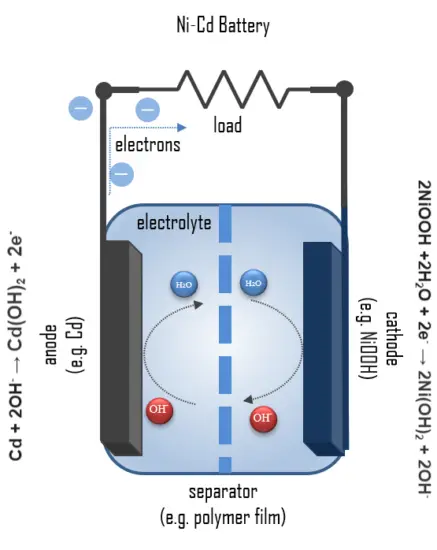
A fully charged Ni-Cd cell contains:
- Cathode: a nickel(III) oxide-hydroxide positive electrode plate
- Anode: a cadmium negative electrode plate
- Separator.
- Electrolyte: an alkaline electrolyte (potassium hydroxide).
Ni-Cd batteries usually have a metal case with a sealing plate equipped with a self-sealing safety valve. The positive and negative electrode plates, isolated from each other by the separator, are rolled in a spiral shape inside the case. This is known as the jelly-roll design and allows a Ni-Cd cell to deliver a much higher maximum current than an equivalent-size alkaline cell.
The positive electrode in the discharged state is composed of nickel hydroxide, which has been doped and modified to meet the battery requirements, and graphite as the conductive medium. The nickel cycles between two oxidation states during charge and discharge; upon the charge, the nickel hydroxide is converted into nickel oxyhydroxide (NiOOH):
2Ni(OH)2 + 2OH- →2NiOOH +2 H2O + 2e–
During discharge, the reactions at the nickel oxide electrode are:
2NiOOH +2H2O + 2e– → 2Ni(OH)2 + 2OH–
The negative electrode consists of cadmium hydroxide, Cd(OH)2, which is reduced to metallic cadmium during charging. The reaction is reversed throughout the discharge process, changing the oxidation state of cadmium from 0 to 2+, releasing two electrons per cadmium atom partaking in the reaction. Below is the reaction for the anode during charge:
Cd(OH)2 + 2e– → Cd + 2OH–
The chemical reactions at the cadmium electrode during discharge are:
Cd + 2OH– → Cd(OH)2 + 2e–
The overall reaction during discharge is:
2NiOOH + Cd + 2H2O → 2Ni(OH)2 + Cd(OH)2
Cadmium is a fairly hazardous metal on its own, therefore, a series of regulations and guidelines on how to handle it are in place by both governments all over the world and the EU.
The electrolyte acts as the ion charge carrier in batteries, and for Ni-Cd batteries, this mainly consists of concentrated potassium hydroxide, KOH, but may also have additions of sodium hydroxide, NaOH, and lithium hydroxide, LiOH.



