30-second summary
How Batteries Work
The fundamental principle in an electrochemical cell is spontaneous redox reactions in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated.
Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals.
When the circuit is closed, the stronger attraction for the electrons by the cathode (e.g. manganese dioxide in alkaline batteries) will pull the electrons from the anode (e.g. zinc) through the wire in the circuit to the cathode electrode. This battery chemical reaction, this flow of electrons through the wire, is electricity.
In simple terms, each battery is designed to keep the cathode and anode separated to prevent a reaction. The stored electrons will only flow when the circuit is closed. This happens when the battery is placed in a device and the device is turned on.
An electric battery is essentially a source of DC electrical energy. How do batteries work? Batteries convert stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. A typical battery consists of one or more voltaic cells.
The fundamental principle in an electrochemical cell is spontaneous redox reactions in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated.
Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals. Even though a wide range of types of batteries exists with different combinations of materials, all of them use the same principle of the oxidation-reduction reaction. Batteries are designed so that the energetically favorable redox reaction can occur only when electrons move through the external part of the circuit.
Batteries are made of an extensive range of materials resulting in different capabilities and behaviors in the functionality of the battery. The most common ones are lead, nickel, and lithium, each of them with different outputs and specific for some different purposes depending on the requirements.
How battery works – Principle of operation
How do batteries work? In simple terms, each battery is designed to keep the cathode and anode separated to prevent a reaction. The stored electrons will only flow when the circuit is closed. This happens when the battery is placed in a device and the device is turned on.
When the circuit is closed, the stronger attraction for the electrons by the cathode (e.g. manganese dioxide in alkaline batteries) will pull the electrons from the anode (e.g. zinc) through the wire in the circuit to the cathode electrode. This battery chemical reaction, this flow of electrons through the wire, is electricity.
If we go into detail, batteries convert chemical energy directly to electrical energy. Chemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals.
Even though a wide range of types of batteries exists with different combinations of materials, all of them use the same principle of the oxidation-reduction reaction. In an electrochemical cell, spontaneous redox reactions take place in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated. The redox reaction is a chemical reaction that produces a change in the oxidation states of the atoms involved. Electrons are transferred from one element to another. As a result, the donor element, which is the anode, is oxidized (loses electrons) and the receiver element, the cathode, is reduced (gains electrons).
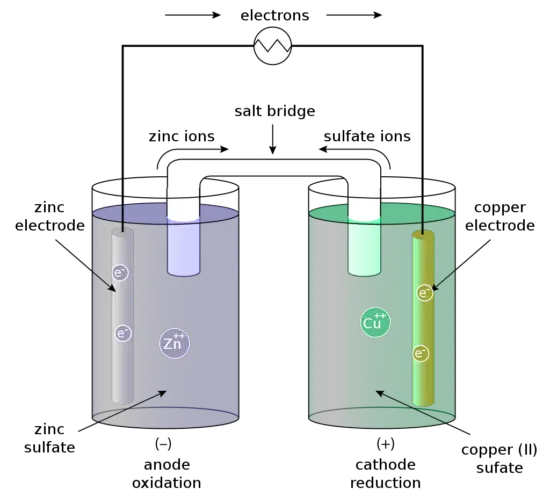
For example, the Daniell cell consists of two electrodes of dissimilar metals, Zn and Cu; each electrode is in contact with a solution of its own ion; Zinc sulfate and copper sulfate respectively. The redox reaction that occurs in the Daniell Cell is:
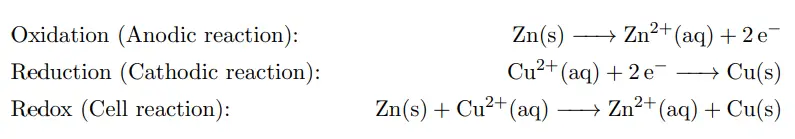
These processes result in the accumulation of solid copper at the cathode and the corrosion of the zinc electrode into the solution as zinc cations. Excess electrons produced by the oxidation of zinc metal are “pushed” out of the anode, which is. Therefore, the negative electrode, travel through the wire and are “pulled” into the copper cathode, where they are consumed by the reduction of copper ions. The electrical current will cause a potential difference and an electromotive force. The spontaneous reaction will occur because zinc is a better reducing agent than copper and thereby more able to emit electrons. A stronger ability to emit electrons at the anode will cause a stronger electromotive force. A salt bridge or ion bridge, in electrochemistry, is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell. It maintains electrical neutrality within the internal circuit.
Lithium-ion battery works
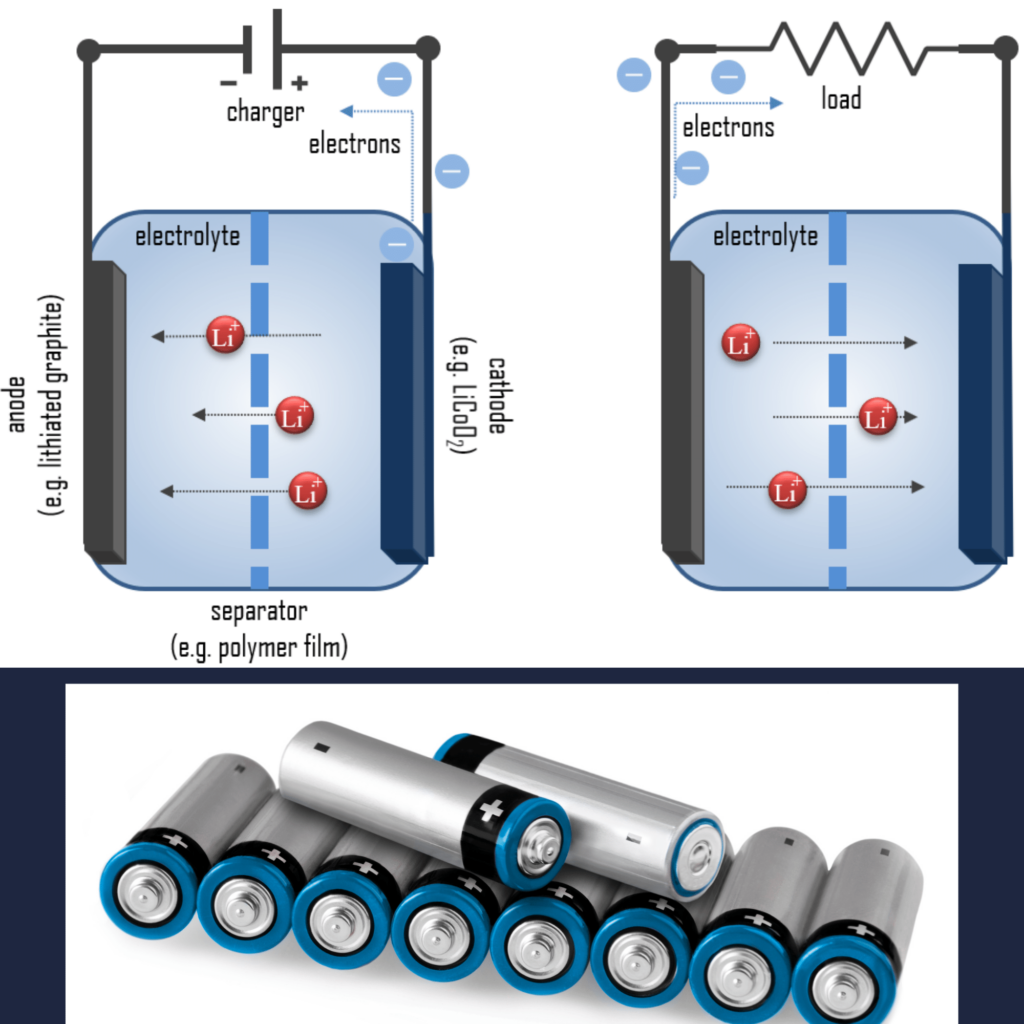
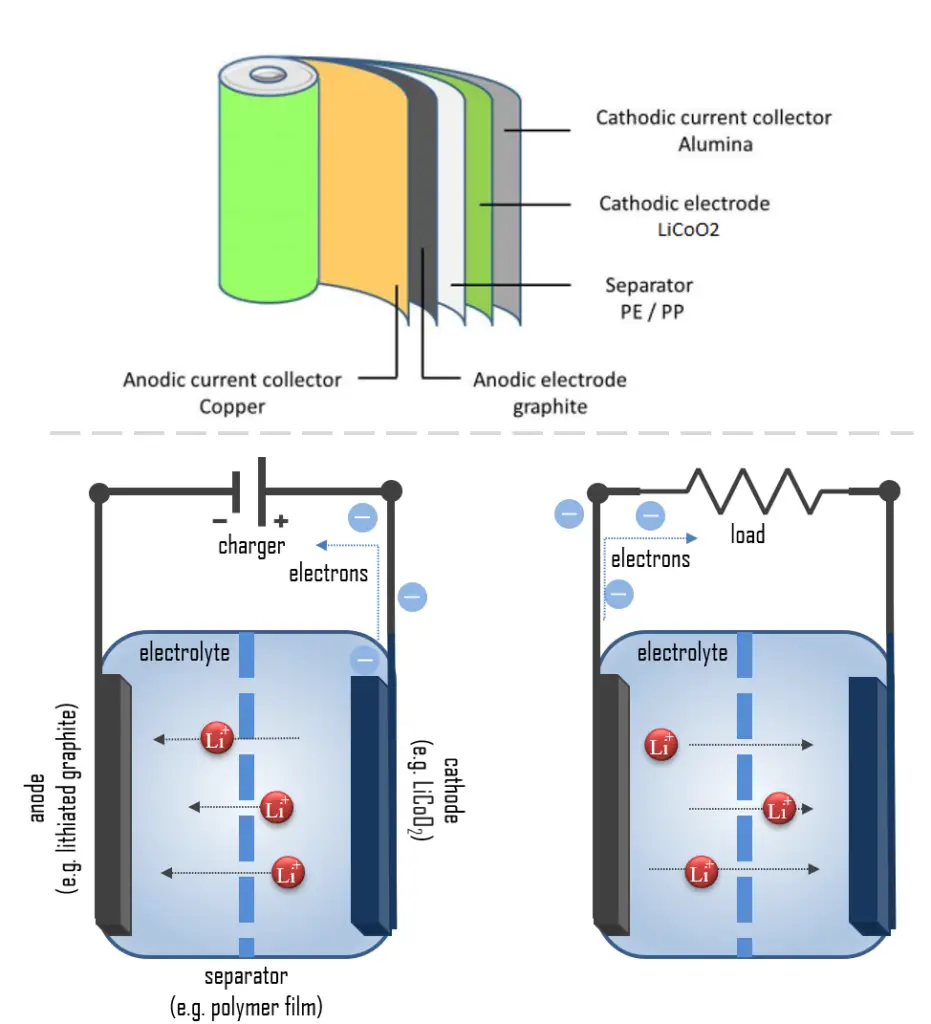
A lithium-ion battery, also known as Li-ion battery, is a type of secondary (rechargeable) battery composed of cells in which lithium ions move from the anode through an electrolyte to the cathode during discharge and back when charging.
How do lithium-ion batteries work? The cathode is made of a composite material (an intercalated lithium compound) and defines the name of the Li-ion battery cell. The anode is usually made out of porous graphite. The electrolyte can be liquid, polymer, or solid. The separator is porous to enable the transport of lithium ions and prevents the cell from short circuiting and thermal runaway.
Lithium metal is the lightest metal and possesses a high specific capacity (3.86 Ah/g) and an extremely low electrode potential (−3.04 V vs. standard hydrogen electrode). Therefore lithium is an ideal anode material for high-voltage and high-energy batteries.
When the circuit is closed, the stronger attraction for the electrons by the cathode (e.g. LiCoO2 in lithium-ion batteries) will pull the electrons from the anode (e.g. lithium-graphite) through the wire in the circuit to the cathode electrode. This battery chemical reaction, this flow of electrons through the wire, is electricity.
During discharge, lithium is oxidized from Li to Li+ (0 to +1 oxidation state) in the lithium-graphite anode through the following reaction:
C6Li → 6C(graphite) + Li+ + e–
These lithium ions migrate through the electrolyte medium to the cathode, where they are incorporated into lithium cobalt oxide through the following reaction, which reduces cobalt from a +4 to a +3 oxidation state:
CoO2 (s) + Li+ + e– → LiCoO2 (s)
Here is the full reaction (left to right = discharging, right to left = charging):
C6Li + CoO2 ⇄ C6 + LiCoO2
These reactions can be run in reverse to recharge the cell. In this case, the lithium ions leave the lithium cobalt oxide cathode and migrate back to the anode, where they are reduced back to neutral lithium and reincorporated into the graphite network.
How alkaline battery works
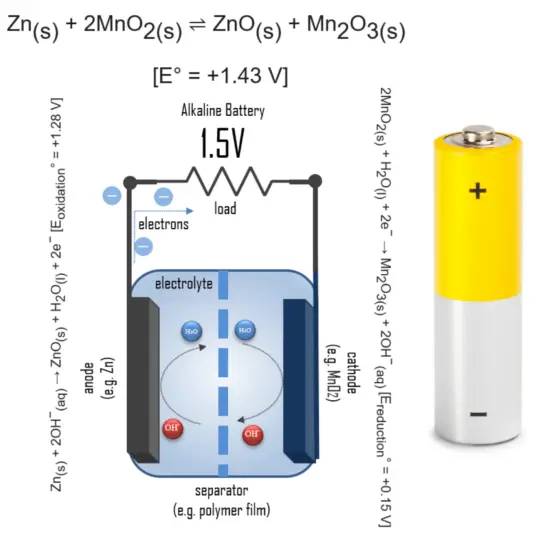
An alkaline battery (IEC code: L) is a type of primary battery that provides direct electric current from the electrochemical reaction between zinc and manganese dioxide (MnO2) in the presence of an alkaline electrolyte.
How do alkaline batteries work? In an alkaline battery, the negative electrode is zinc, and the positive electrode is high-density manganese dioxide (MnO2). The alkaline electrolyte of potassium hydroxide, KOH, is not consumed during the reaction. Only zinc and MnO2 are consumed during discharge. The alkaline electrolyte of potassium hydroxide remains, as there are equal amounts of OH− consumed and produced. When the circuit is closed, the stronger attraction for the electrons by the cathode (e.g. manganese dioxide in alkaline batteries) will pull the electrons from the anode (e.g. zinc) through the wire in the circuit to the cathode electrode. This battery chemical reaction, this flow of electrons through the wire, is electricity.
The half-reactions are:
Zn(s) + 2OH−(aq) → ZnO(s) + H2O(l) + 2e− [Eoxidation° = +1.28 V]
2MnO2(s) + H2O(l) + 2e− → Mn2O3(s) + 2OH−(aq) [Ereduction° = +0.15 V]
Overall reaction:
Zn(s) + 2MnO2(s) ⇌ ZnO(s) + Mn2O3(s) [e° = +1.43 V]
Applying this battery chemistry to the real world, the electrons generated during the reaction are used to power devices when the circuit is closed.
This is simply how batteries work.