Lithium-ion Battery
A lithium-ion battery, also known as the Li-ion battery, is a type of secondary (rechargeable) battery composed of cells in which lithium ions move from the anode through an electrolyte to the cathode during discharge and back when charging.
The cathode is made of a composite material (an intercalated lithium compound) and defines the name of the Li-ion battery cell. The anode is usually made out of porous lithiated graphite. The electrolyte can be liquid, polymer, or solid. The separator is porous to enable the transport of lithium ions and prevents the cell from short-circuiting and thermal runaway.
Chemistry, performance, cost, and safety characteristics vary across types of lithium-ion batteries. Handheld electronics mostly use lithium polymer batteries (with a polymer gel as electrolyte), a lithium cobalt oxide (LiCoO2) cathode material, and a graphite anode, which offer high energy density.
Li-ion batteries, in general, have a high energy density, no memory effect, and low self-discharge. One of the most common types of cells is 18650 battery, which is used in many laptop computer batteries, cordless power tools, certain electric cars, electric kick scooters, most e-bikes, portable power banks, and LED flashlights. The nominal voltage is 3.7 V.
Note that non-rechargeable primary lithium batteries (like lithium button cells CR2032 3V) must be distinguished from secondary lithium-ion or lithium-polymer, which are rechargeable batteries. Primary lithium batteries contain metallic lithium, which lithium-ion batteries do not.
Lithium-ion vs Lithium polymer batteries
A lithium-ion polymer (LiPo) battery (also known as Li-pol, lithium-poly, and other names) is a type of Li-ion battery with a polymer electrolyte instead of a liquid electrolyte. All LiPo batteries use a high-conductivity gel polymer as the electrolyte. Lithium polymer cells have evolved from lithium-ion and lithium-metal batteries. The primary difference between lithium-ion and Li-pol is that instead of using a liquid lithium-salt electrolyte (such as LiPF6) held in an organic solvent, the battery uses a solid polymer electrolyte (SPE) such as polyethylene oxide (PEO), polyacrylonitrile (PAN), polymethyl methacrylate (PMMA) or polyvinylidene fluoride (PVdF). LiPos provide higher specific energies than other lithium batteries, often used in systems where weight is an important factor, such as mobile devices, drones, and some electric vehicles.
Composition of Lithium-ion Batteries
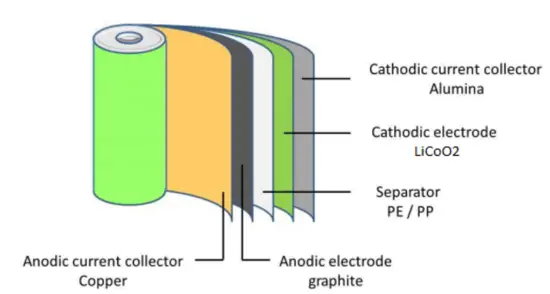
A typical lithium-ion cell contains:
- Cathode: The cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction. In the case of lithium batteries, cathode materials are generally constructed from LiCoO2 or LiMn2O4. For the cathode, it is important to hold a large amount of lithium without significant change in structure, have good chemical and electrochemical stability with electrolyte, be a good electrical conductor and diffuser of lithium ions, and be of low cost.
- Anode: The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during an electrochemical reaction. One of the most common anode materials used today is lithiated graphite, LixC6, which is composed of graphite sheets intercalated with lithium. New materials, such as those based on Silicon and other elemental blends, are being researched. Lithiated graphite has a unit cell with an HCP structure.
- Separator. A separator is a permeable membrane placed between a battery’s anode and cathode. The main function of a separator is to keep the two electrodes apart to prevent electrical short circuits while also allowing the transport of ionic charge carriers that are needed to close the circuit during the passage of current in an electrochemical cell. Commercially available liquid electrolyte cells use microporous polyolefin materials, such as polyethylene (PE) or polypropylene (PP). Separators in Li-ions have to be electrochemically and chemically stable relative to the electrolyte and electrode materials. Functional separators that use MOF-coated membranes to perform the dual functions of the electrolyte and separator are being developed to support the design of high-performance Li-metal batteries for high-energy systems in electric vehicles and electric aircraft.
- Electrolyte: The choice of electrolyte in all batteries is critical for performance as well as safety. Most of the electrolytes used in commercial lithium-ion batteries are non-aqueous solutions, in which Lithium hexafluorophosphate (LiPF6) salt dissolved in organic carbonates, in particular, mixtures of ethylene carbonate (EC) with dimethyl carbonate (DMC), propylene carbonate (PC), diethyl carbonate (DEC), and/or ethyl methyl carbonate (EMC). A good electrolyte must have low reactivity with other cell components, high ionic conductivity, low toxicity, a large window of electrochemical voltage stability (0-5V), and be thermally stable.

