The zinc-carbon battery, also called the Leclanché cell, is a traditional general-purpose dry cell. Zinc–carbon batteries were the first commercial dry batteries developed from the technology of the wet Leclanché cell. This battery provides a direct electric current from the electrochemical reaction between zinc and manganese dioxide (MnO2) in the presence of an electrolyte.
Its name comes from the cathode, which is a mixture of powdered carbon (usually graphite powder) and manganese (IV) oxide (MnO2), which is packed around a carbon rod.
The zinc-carbon cell has an electrolyte of ammonium chloride or zinc chloride, which is dissolved in water. Zinc-carbon batteries today have been mostly replaced by more efficient and safe alkaline batteries. It produces a voltage of about 1.5 volts between the zinc anode, which is typically constructed as a cylindrical container for the battery cell, and a carbon rod surrounded by the cathode that collects the current from the manganese dioxide electrode. The electrolyte consists of a saturated aqueous solution of ammonium chloride containing roughly 20 percent zinc chloride. The name “zinc-carbon” is slightly misleading as it implies that carbon is acting as the reducing agent rather than the manganese dioxide.
In the United States, the alkaline zinc-manganese dioxide (Zn- MnO2 ) has generally replaced all the Leclanche cells. Compared to the modern alkaline cells, Leclanche cells have the following disadvantages:
- Leclanche cells are not suitable for high-rate continuous discharge
- The capacity of Leclanche cells is much lower than modern alkaline cells
- The leakage phenomenon is common in Leclanche cells.
Advantages and Disadvantages of Primary Batteries
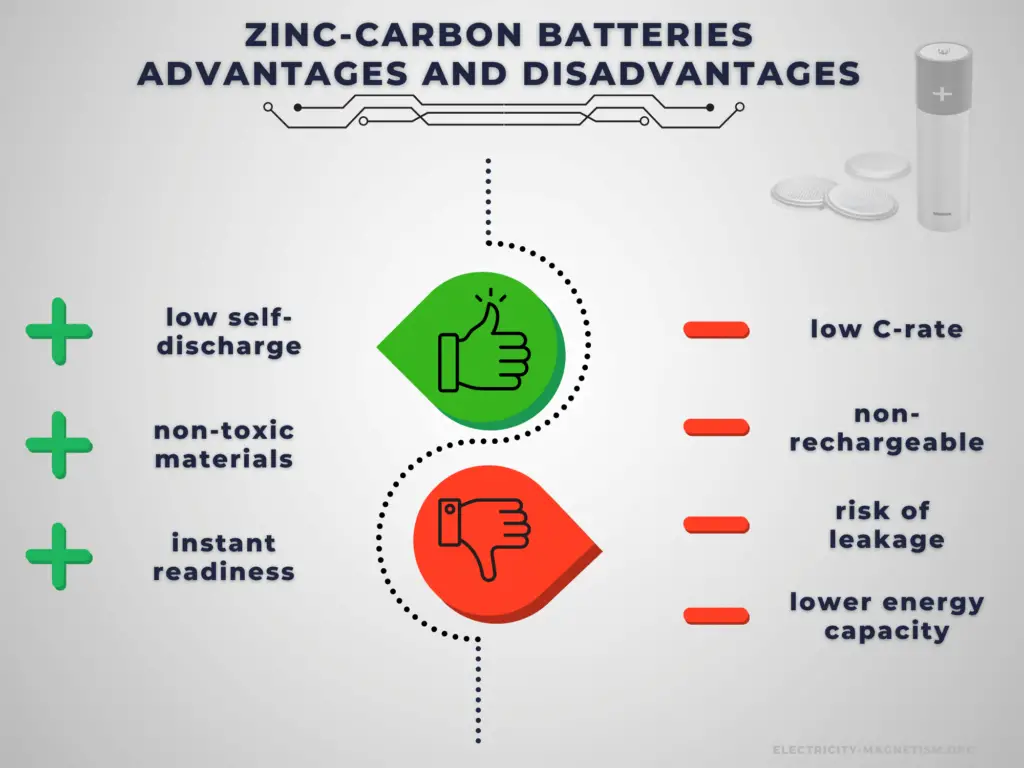
Advantages:
Primary cells have higher energy density than rechargeable secondary cells. High specific energy, long storage times (low self-discharge), and instant readiness give primary batteries a unique advantage over other power sources. They are usually the best choice for low-drain applications. They can be carried to remote locations and used instantly, even after long storage; they are also readily available and environmentally friendly when disposed.
Disadvantages:
The main disadvantage of primary batteries is that they are non-rechargeable. Another disadvantage is their low C-rate. Even high current types are considered low in comparison to rechargeable batteries. They are also less environment friendly than rechargeable batteries. The application of primary batteries leads to a large amount of waste batteries to be recycled. For large batteries, primary batteries are usually not cost-effective.

