Activated carbon supercapacitors are energy storage devices offering high power density, rapid charging, and excellent cycling stability.
Introduction to Supercapacitors
Supercapacitors, also known as ultracapacitors or electrochemical capacitors, are energy storage devices that have seen a surge in interest due to their high power density, excellent cycling stability, and rapid charging capabilities. They function on the principle of electrostatic double-layer capacitance (EDLC) or pseudocapacitance, or a combination of both, storing energy by either physical or chemical means.
EDLC-based supercapacitors utilize charge separation in a Helmholtz double layer at the interface between the surface of a conductive electrode and an electrolyte. On the other hand, pseudocapacitance supercapacitors involve redox reactions, electron charge-transfer, or intercalation processes on the electrode surface. Regardless of the mechanisms, the choice of electrode material is critical in supercapacitors as it directly impacts their energy and power densities.
Activated Carbon as Supercapacitor Electrodes
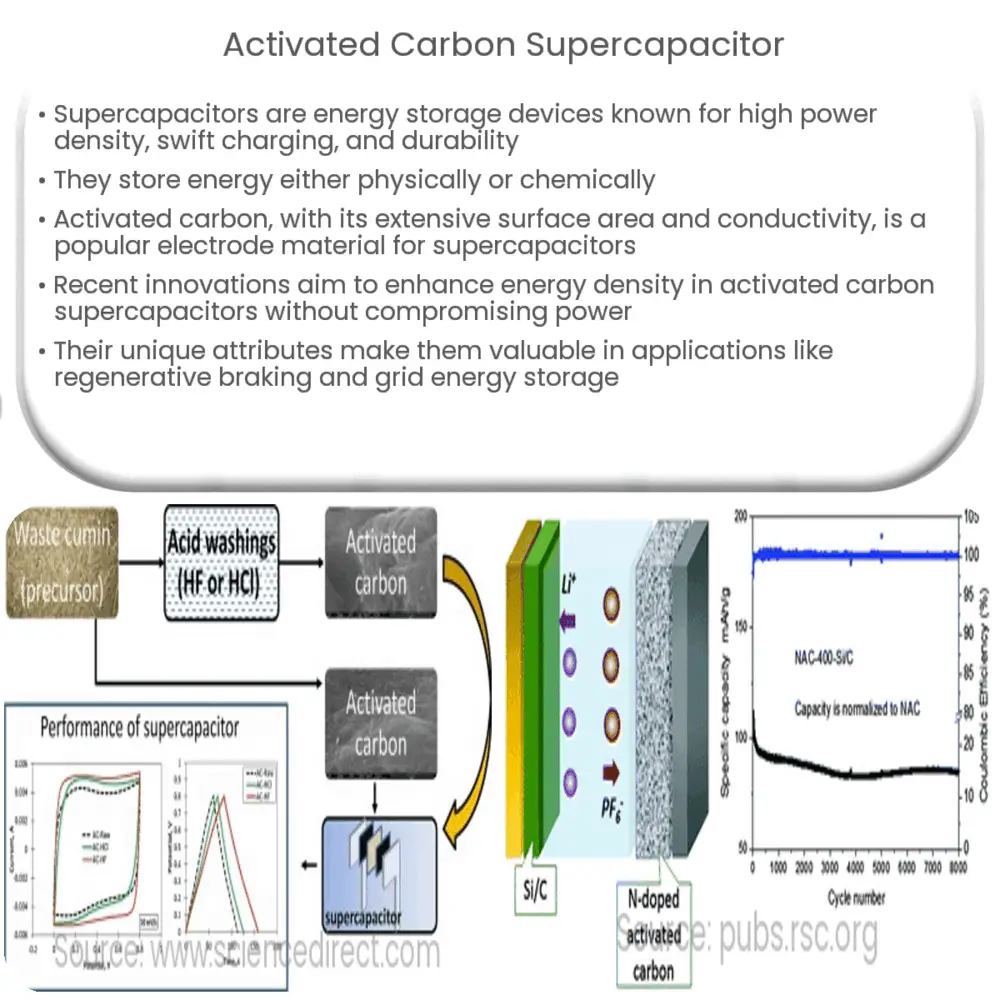
Among the myriad of materials examined for supercapacitor electrodes, activated carbon stands out due to its excellent electrical conductivity, high surface area, good chemical stability, and relatively low cost. Activated carbon is a form of carbon that has been processed to have small, low-volume pores that increase the surface area available for adsorption or chemical reactions. As a result, this material is well-suited to serve as an electrode in a supercapacitor.
The high surface area of activated carbon allows for a larger electric double layer to form, and consequently, a higher energy storage capacity. With surface areas typically exceeding 1000 m²/g, activated carbon can significantly boost the energy storage capacity of a supercapacitor. Moreover, its excellent electrical conductivity ensures minimal energy loss during charge/discharge cycles, contributing to the high power density of supercapacitors.
Activated carbon can be derived from various carbonaceous precursors, including coal, coconut shells, and biomass, through physical or chemical activation processes. These processes create a highly porous structure, offering a vast area for charge accumulation. This characteristic, along with its affordability and availability, make activated carbon a popular choice for supercapacitor electrodes.
While activated carbon supercapacitors excel in power delivery and cycle life, they typically lag behind batteries in terms of energy density. This trade-off between energy density and power density is a crucial consideration in the design and application of energy storage systems. The next section will delve into the recent advancements aimed at enhancing the energy density of activated carbon supercapacitors without sacrificing their power density and cycle life.
Advancements in Activated Carbon Supercapacitors
Recent developments in activated carbon supercapacitors aim to enhance their energy density while preserving their inherent high power density and long cycle life. One such advancement is the optimization of pore size and distribution in activated carbon materials. Studies have shown that the energy density of supercapacitors can be significantly increased by matching the pore size of the activated carbon electrode to the ion size of the electrolyte. This matching maximizes the surface area accessible to electrolyte ions, thus boosting the energy storage capacity.
Another significant breakthrough involves using hybrid supercapacitors, also known as asymmetric supercapacitors. These devices employ an activated carbon electrode paired with a battery-type electrode, typically made of a transition metal oxide or conducting polymer. The activated carbon electrode provides a high power density and excellent cycle stability, while the battery-type electrode contributes to a higher energy density. As a result, hybrid supercapacitors can achieve a higher energy density than traditional activated carbon supercapacitors, without compromising their power density.
Activated Carbon Supercapacitors in Applications
The unique properties of activated carbon supercapacitors have paved the way for their use in a wide range of applications. Their high power density and excellent cycling stability make them ideal for applications that require quick bursts of energy, such as in regenerative braking systems in electric vehicles. Furthermore, their rapid charging capability can also be leveraged in energy-harvesting devices, such as solar and wind power systems, where they can store energy quickly during peak generation times and release it as needed.
Moreover, the long cycle life of activated carbon supercapacitors makes them well-suited for applications where long-term reliability is crucial, such as in grid energy storage. They can endure millions of charge/discharge cycles without significant degradation, making them a promising solution for grid storage where stability over long periods is paramount.
In conclusion, the versatility of activated carbon supercapacitors, coupled with the ongoing advancements in their design and fabrication, positions them as a significant player in the future of energy storage solutions. Their unique blend of high power density, excellent cycling stability, and rapid charging capability offers a promising route to addressing the energy challenges of the future.

