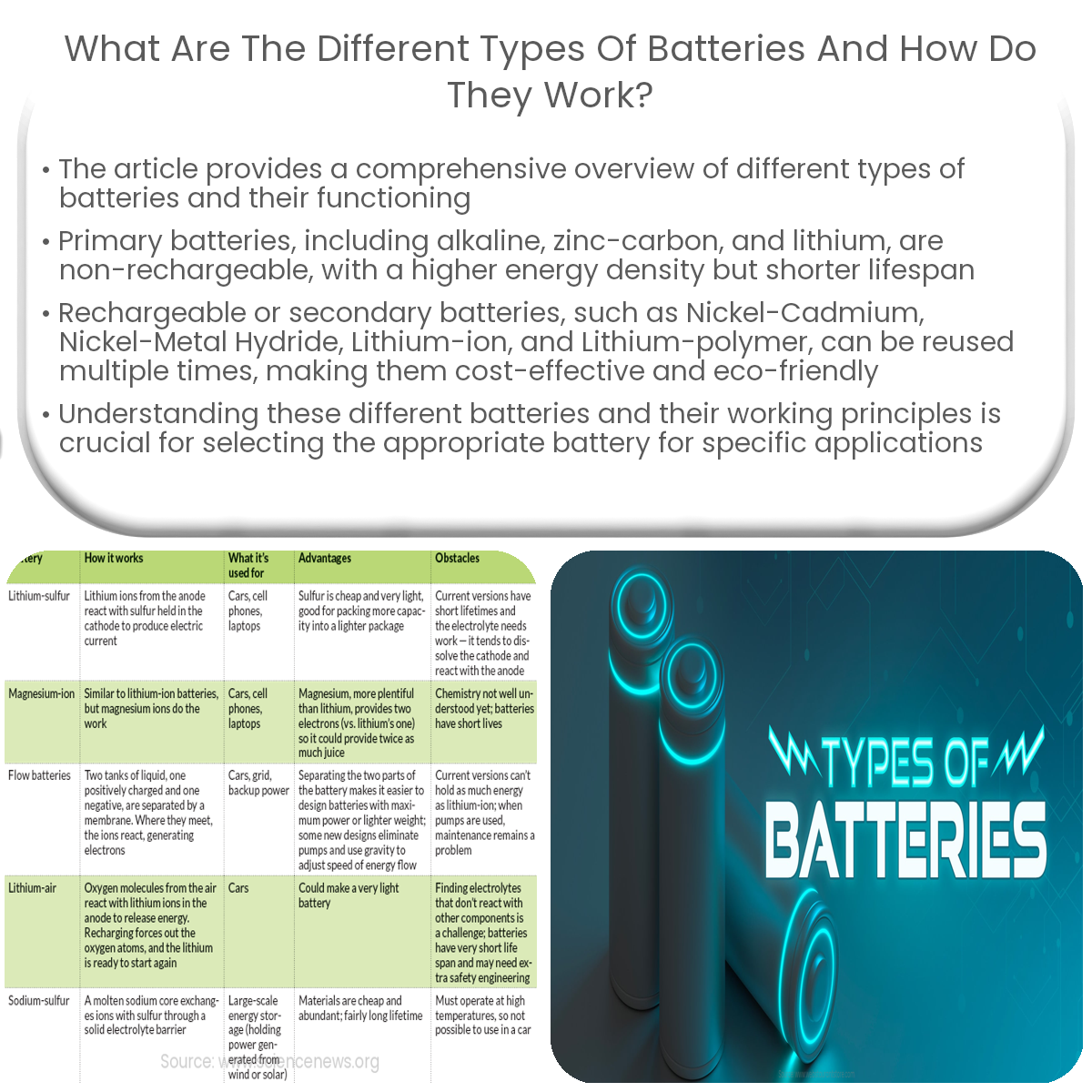
Different battery types include alkaline, zinc-carbon, and lithium (primary), and nickel-cadmium, nickel-metal hydride, lithium-ion, and lithium-polymer (rechargeable).
Understanding Different Types of Batteries and Their Functioning
Batteries are essential for powering a wide range of devices, from smartphones to electric vehicles. This article will provide an overview of the different types of batteries and how they work.
Primary Batteries
Primary batteries are non-rechargeable and must be discarded after they are depleted. They typically have a higher energy density but a shorter lifespan compared to rechargeable batteries. Common types of primary batteries include:
- Alkaline batteries: Widely used in household items like remote controls and flashlights, these batteries rely on a chemical reaction between zinc and manganese dioxide to produce electricity.
- Zinc-carbon batteries: These are less expensive than alkaline batteries but have a lower energy density. Zinc and manganese dioxide are used as electrodes, while ammonium chloride or zinc chloride serves as the electrolyte.
- Lithium batteries: Offering high energy density and long shelf life, lithium batteries are used in applications like cameras and smoke detectors. They generate electricity through the reaction between lithium metal and a variety of compounds.
Rechargeable Batteries
Rechargeable batteries, also known as secondary batteries, can be recharged multiple times, making them cost-effective and environmentally friendly. Some common rechargeable batteries are:
- Nickel-Cadmium (NiCd) batteries: These batteries have a long life cycle and can deliver high discharge rates. However, they suffer from the “memory effect” and contain toxic cadmium, limiting their use in consumer products.
- Nickel-Metal Hydride (NiMH) batteries: With higher energy density than NiCd batteries and less environmental impact, NiMH batteries are commonly used in consumer electronics. They do have a higher self-discharge rate and can be sensitive to high temperatures.
- Lithium-ion (Li-ion) batteries: These batteries have a high energy density, low self-discharge rate, and no memory effect. Widely used in smartphones, laptops, and electric vehicles, Li-ion batteries rely on the movement of lithium ions between the positive and negative electrodes during charging and discharging.
- Lithium-polymer (Li-Po) batteries: Similar to Li-ion batteries, Li-Po batteries use a polymer electrolyte, making them lightweight and flexible. They are used in applications like drones and wearable devices but can be more expensive and have a shorter lifespan than Li-ion batteries.
Conclusion
Understanding the different types of batteries and their working principles is crucial for selecting the right battery for a specific application. Primary batteries are ideal for single-use devices, while rechargeable batteries offer long-term cost savings and reduced environmental impact for devices requiring frequent charging and discharging.