Quartz crystal microbalance (QCM) is a sensitive technique for analyzing thin films and molecular interactions in various research fields.
Quartz Crystal Microbalance (QCM): A Powerful Tool for Analyzing Thin Films and Molecular Interactions
Introduction
Quartz crystal microbalance (QCM) is a highly sensitive and versatile technique used for measuring minute mass changes in the nanogram range. This powerful tool has gained significant popularity in various fields, including chemistry, biology, materials science, and engineering, due to its ability to analyze thin films and study molecular interactions. In this article, we will discuss the fundamentals of QCM, its working principle, and its applications in various research areas.
Principle of Quartz Crystal Microbalance
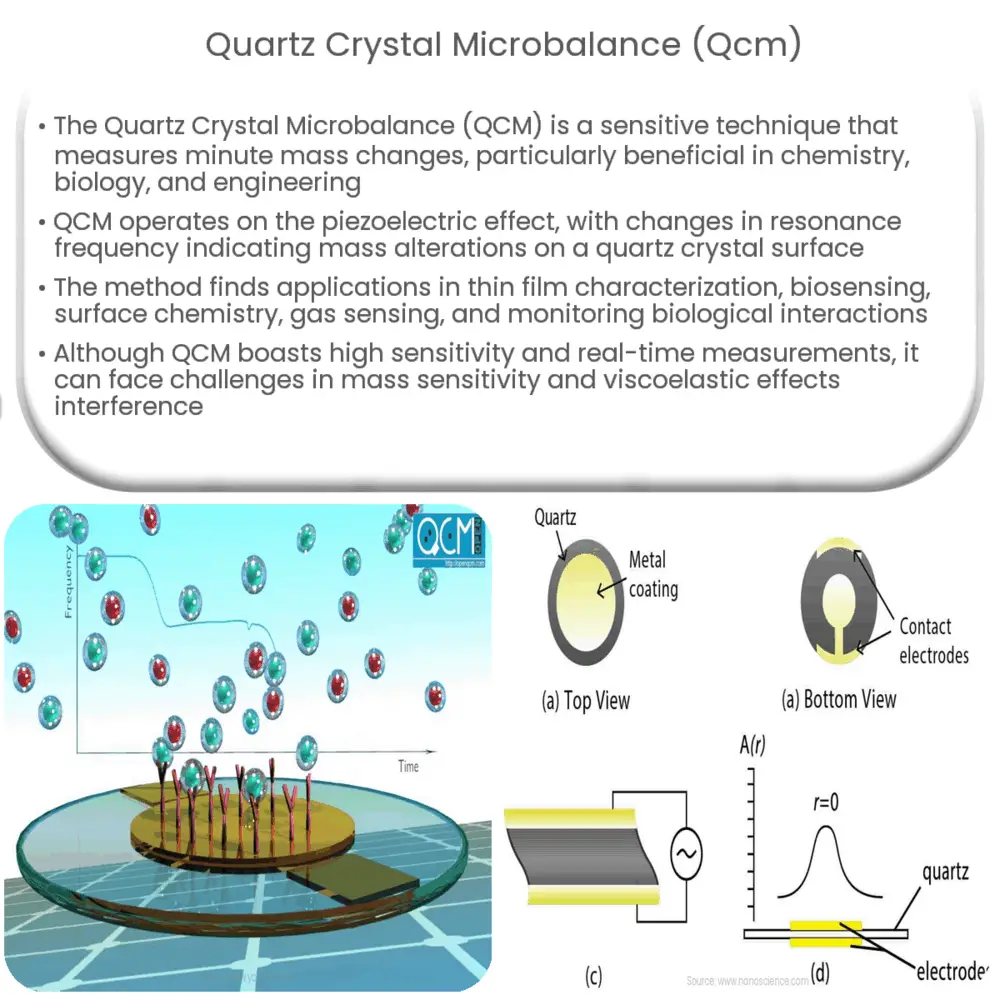
QCM is based on the piezoelectric effect, a phenomenon in which certain materials generate an electric charge when subjected to mechanical stress or, conversely, undergo mechanical deformation when exposed to an electric field. Quartz, a naturally occurring crystalline mineral, is an excellent piezoelectric material and forms the basis for QCM technology.
At the heart of QCM is a thin, disk-shaped quartz crystal, typically with a gold or silver electrode coating on both sides. When an alternating voltage is applied to these electrodes, the crystal oscillates at a specific frequency, known as its resonance frequency. This oscillation frequency is sensitive to the mass changes on the surface of the crystal, which allows QCM to function as a highly accurate mass sensor.
Working Mechanism of QCM
QCM operates by monitoring the changes in the resonance frequency of the quartz crystal as a function of mass added or removed from its surface. When a thin film or molecular layer is deposited onto the crystal, the added mass causes a decrease in the oscillation frequency. This change in frequency is directly proportional to the mass change, as described by the Sauerbrey equation:
Δf = -C * Δm/A
Here, Δf is the change in resonance frequency, Δm is the change in mass, A is the active area of the crystal, and C is the sensitivity constant specific to the crystal. By measuring the frequency shift and using the Sauerbrey equation, researchers can determine the mass of the deposited material with high accuracy and precision.
Applications of QCM
QCM has found widespread use in various research fields and industries, thanks to its high sensitivity, real-time measurements, and compatibility with different environments. Some common applications of QCM include:
- Thin Film Characterization: QCM is an excellent tool for characterizing thin films, such as polymer coatings, metal layers, and biomolecular films. Researchers can study film thickness, uniformity, and viscoelastic properties using QCM.
- Biosensing: By functionalizing the QCM surface with specific recognition elements, such as antibodies or aptamers, QCM can be employed as a highly sensitive biosensor for detecting target molecules, including proteins, nucleic acids, and small molecules.
- Surface Chemistry and Catalysis: QCM allows researchers to investigate surface reactions, adsorption, and desorption processes in real-time. This capability has proven particularly useful in the study of catalytic materials and their mechanisms.
- Gas Sensing: QCM-based sensors have been developed to detect various gases and vapors, including volatile organic compounds (VOCs), humidity, and toxic gases. These sensors offer high sensitivity, selectivity, and rapid response times.
- Monitoring of Biological Interactions: QCM can be employed to investigate biomolecular interactions, such as protein-protein, protein-DNA, and antigen-antibody binding. By monitoring the frequency changes during these interactions, researchers can determine binding kinetics, affinity constants, and thermodynamic parameters.
Advantages and Limitations of QCM
QCM offers several advantages as a versatile analytical technique:
- High Sensitivity: QCM is capable of detecting mass changes in the nanogram range, making it suitable for studying very small mass variations.
- Real-time Measurements: QCM provides real-time information on mass changes, enabling researchers to monitor processes as they occur.
- Compatibility: QCM can be used in various environments, including gas, liquid, and vacuum conditions, making it a versatile technique for a wide range of applications.
- Non-destructive Analysis: As QCM is a label-free technique, it does not require any modifications or alterations to the sample, preserving its integrity during the analysis.
However, QCM also has some limitations:
- Limitations in Mass Sensitivity: While QCM is highly sensitive, it is not suitable for measuring mass changes below its detection limit, which might be an issue for some applications.
- Interference from Viscoelastic Effects: In certain cases, viscoelastic properties of the deposited material can cause deviations from the Sauerbrey equation, complicating data analysis and interpretation.
Conclusion
Quartz crystal microbalance (QCM) is a powerful and versatile technique for analyzing thin films and studying molecular interactions. Its high sensitivity, real-time measurements, and compatibility with different environments make it an invaluable tool in various research fields and industries. While QCM has some limitations, its numerous advantages and wide range of applications continue to attract researchers and drive new innovations in this exciting field.

