Explore the advancements and potential of ionic liquid supercapacitors, a promising solution for sustainable, high-capacity energy storage.
Introduction to Ionic Liquid Supercapacitors
Supercapacitors, also known as ultracapacitors, have gained significant attention in recent years due to their superior power density and long cycle life compared to traditional batteries. Their applicability ranges from portable electronics to electric vehicles and grid energy storage. However, one challenge remains – enhancing their energy density. This is where ionic liquid supercapacitors come into play.
Ionic Liquids: A Key Component
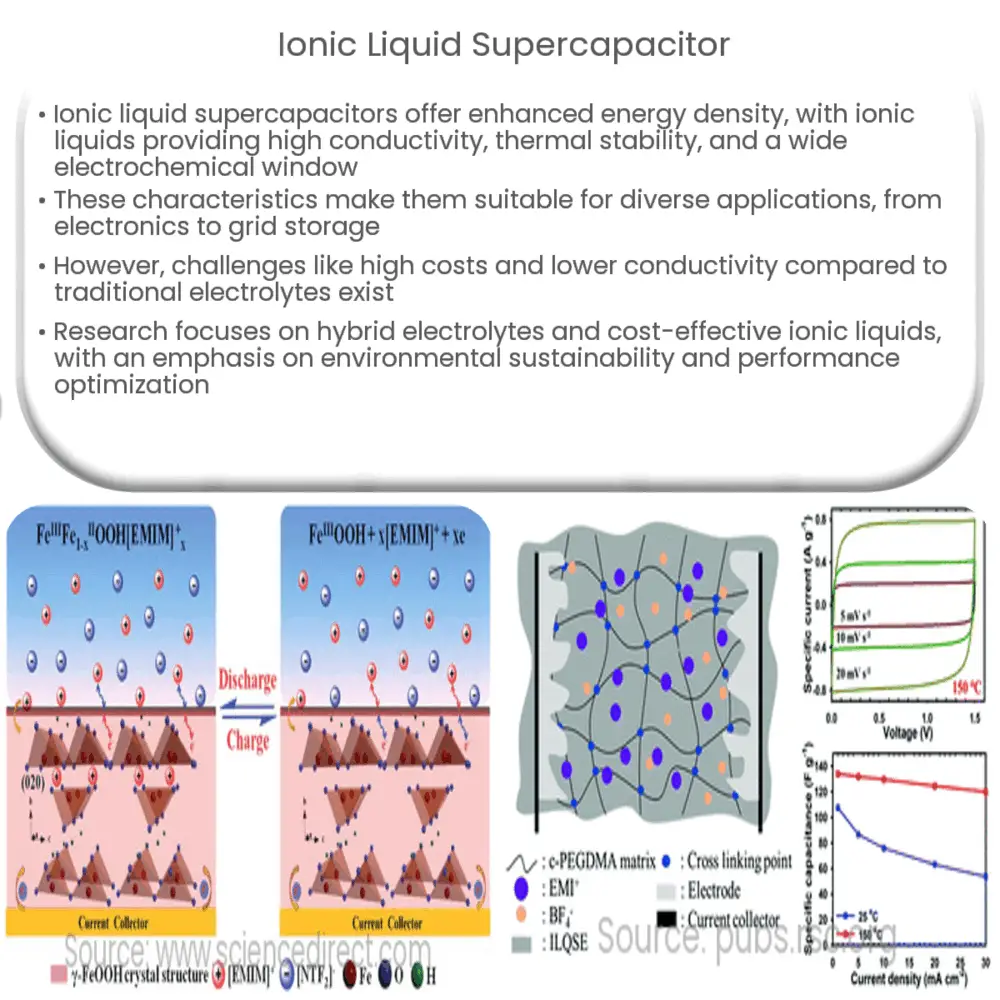
The primary element in these novel supercapacitors is ionic liquids. These are salts in a liquid state, which exist under room temperature. The unique properties of ionic liquids such as high conductivity, wide electrochemical window, and excellent thermal stability make them an ideal choice as electrolytes for supercapacitors.
- High Conductivity: Ionic liquids, owing to their ionic nature, exhibit high electrical conductivity, which is essential for efficient energy storage and discharge.
- Wide Electrochemical Window: This refers to the voltage range within which the ionic liquid remains stable without decomposing. A wider window allows for a higher energy storage capacity.
- Excellent Thermal Stability: Ionic liquids can withstand high temperatures without breaking down, making them safer than traditional electrolytes.
Supercapacitors and Ionic Liquids
The integration of ionic liquids into supercapacitors brings several benefits. Firstly, it significantly enhances the energy storage capacity due to the wide electrochemical window of ionic liquids. Additionally, they offer the ability to operate under a wider range of temperatures, making them suitable for harsh environmental conditions.
However, despite these potential advantages, there are still a few challenges that need to be addressed before ionic liquid supercapacitors can be commercialized. These include the high cost of ionic liquids and their relatively lower conductivity compared to traditional organic electrolytes.
Research and Developments
There has been a plethora of research focusing on these hurdles. For instance, researchers are exploring the use of hybrid electrolytes that combine ionic liquids with conventional electrolytes to balance conductivity and cost. Furthermore, the development of new, cost-effective ionic liquids is a burgeoning area of research.
Progress in Ionic Liquid Supercapacitors
Despite the challenges, significant progress has been made in the development of ionic liquid supercapacitors. A key focus of recent studies has been to find the optimal combination of ionic liquid electrolytes and electrode materials. The goal is to maximize the energy density without compromising on the other essential supercapacitor properties such as power density and cycle life.
Another promising approach has been the development of composite electrolytes. In these systems, ionic liquids are combined with other materials like polymers or ceramics. These composites can potentially provide the best of both worlds – the high conductivity and electrochemical stability of ionic liquids, and the mechanical strength and lower cost of the other materials.
Environmental Impact and Sustainability
Notably, ionic liquid supercapacitors also offer environmental benefits. Ionic liquids are generally less toxic and more biodegradable than conventional organic electrolytes, making them a more sustainable choice. This is particularly important given the growing demand for energy storage solutions and the consequent environmental impact of their production and disposal.
Future Perspectives
Looking forward, the potential of ionic liquid supercapacitors is vast. They could revolutionize energy storage across a wide range of applications, from portable electronics to grid energy storage and electric vehicles. However, more research is required to further optimize these systems and to make them economically competitive with existing technologies. As our understanding of ionic liquids and their interaction with electrode materials improves, so too will the performance and cost-effectiveness of these supercapacitors.
Conclusion
In conclusion, ionic liquid supercapacitors represent a promising avenue for the future of energy storage. While challenges persist, the ongoing research and developments in this field are encouraging. The superior properties of ionic liquids, such as high thermal stability and a wide electrochemical window, combined with their environmental benefits, make them an attractive option for the next generation of supercapacitors. As we continue to innovate and seek sustainable solutions, ionic liquid supercapacitors may well become a key player in meeting our ever-growing energy demands.



