30-second summary
Laws of Electrostatics
The First Law of Electrostatics states:
“Like charges of electricity repel each other, whereas unlike charges attract each other.”
According to the Second Law of Electrostatics the force exerted between two point charges:
- is directly proportional to the product of their strengths,
- is inversely proportional to the square of the distance between them,
- is inversely proportional to the absolute permittivity of the surrounding medium.
Laws of Electrostatics
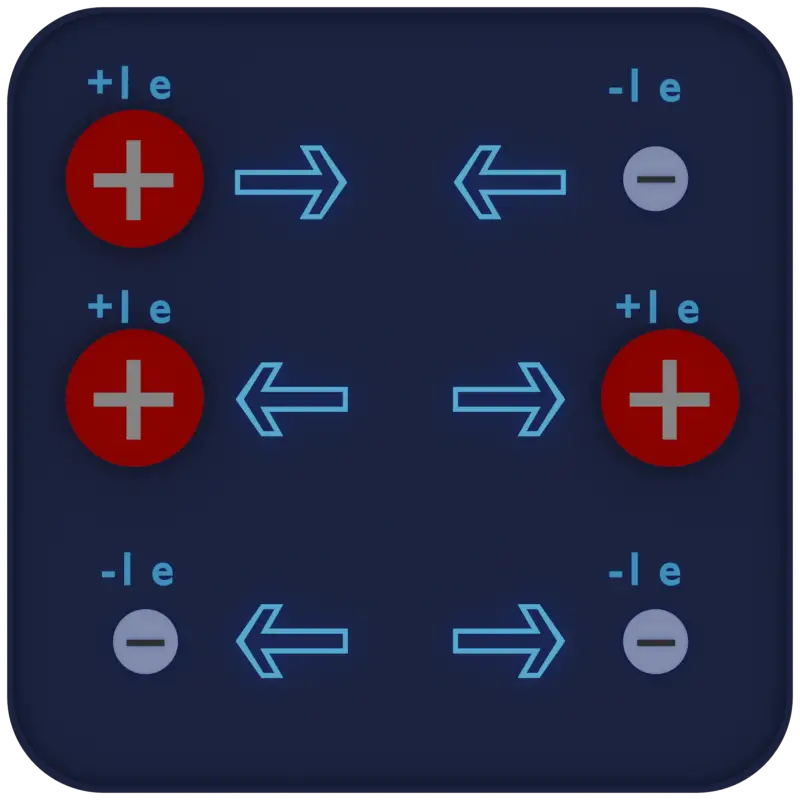
The First Law of Electrostatics states:
“Like charges of electricity repel each other, whereas unlike charges attract each other.”
The negative charge of the electron is equal, but opposite to, the positive charge of the proton. These charges are referred to as electrostatic charges. In nature, unlike charges (like electrons and protons) attract each other, and like charges repel each other. These facts are known as the First Law of Electrostatics and are sometimes referred to as the law of electrical charges.
According to the Second Law of Electrostatics the force exerted between two point charges:
- is directly proportional to the product of their strengths,
- is inversely proportional to the square of the distance between them,
- is inversely proportional to the absolute permittivity of the surrounding medium.
This is known as the Coulomb’s Law.
Frequently asked questions
An atom consists of a positively charged nucleus surrounded by negatively charged electrons so that the atom as a whole is electrically neutral. The atomic nucleus consists of positively charged protons and neutral neutrons.
The electrons in an atom are attracted to the protons in the nucleus by the electromagnetic force. This force binds the electrons inside an electrostatic potential well surrounding the smaller nucleus, which means that an external source of energy is needed for the electron to escape.
The coulomb (symbol: C) is the International System of Units (SI) unit of electric charge. The coulomb was defined as the quantity of electricity transported in one second by a current of one ampere: 1 C = 1 A × 1 s

