30-second summary
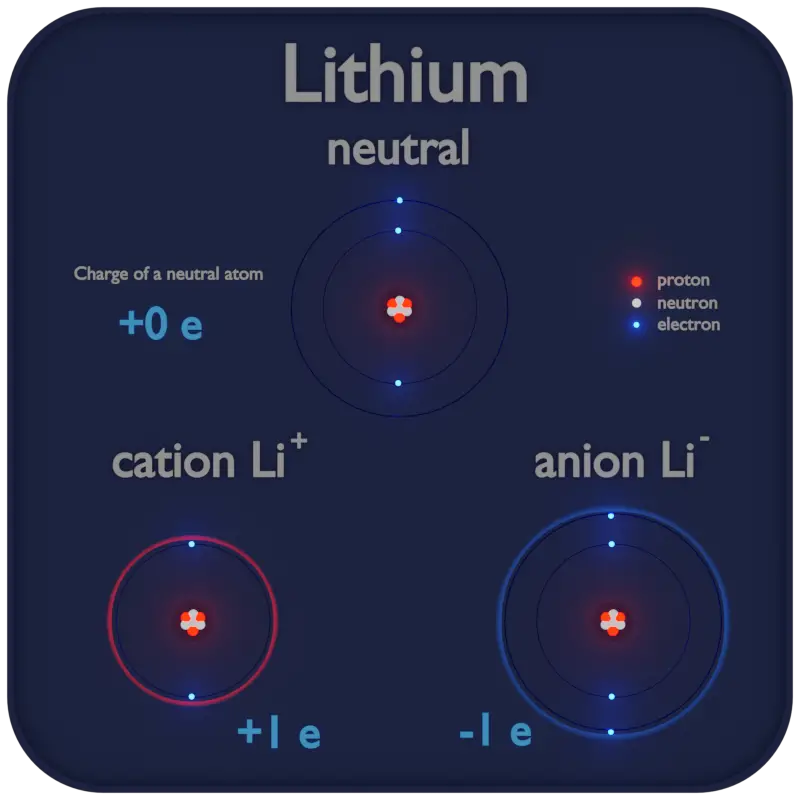
Cation – Positively Charged Atom
An ion is an atom or molecule which has either lost one or more of its electrons or gained one or more electrons. It simply has a net electric charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention.
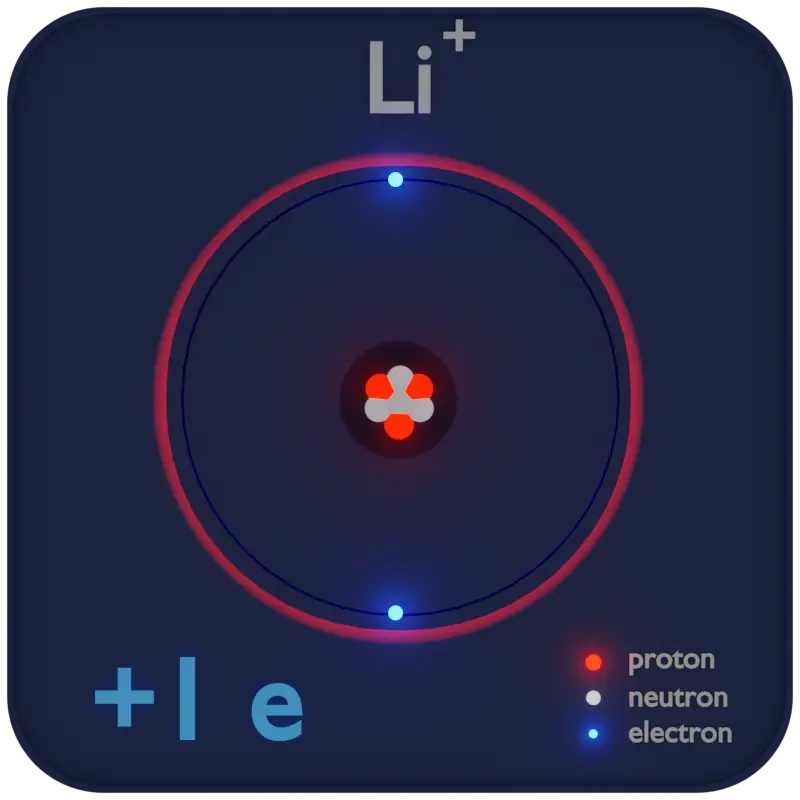
- Cation. A cation is a positively charged ion with fewer electrons than protons.
- Anion. An anion is a negatively charged ion with more electrons than protons.
About Cation
An ion is an atom or molecule which has either lost one or more of its electrons or gained one or more electrons. It simply has a net electric charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention.
- Cation. A cation is a positively charged ion with fewer electrons than protons.
- Anion. An anion is a negatively charged ion with more electrons than protons.
Cations and anions are measured by their ionic radius and they differ in relative size. Cations are small, most of them less than 10−10 m (10−8 cm) in radius. In general, anions are larger than the corresponding neutral atom, since adding electrons increases the number of electron-electron repulsion interactions that take place. Cations are smaller than the corresponding neutral atoms, since the valence electrons, which are furthest away from the nucleus, are lost.
Particles with the same sign of electrical charge repel each other, and particles with opposite signs attract each other. In materials, these oppositely charged ions attract each other to form ionic bond. The electrons in an atom are attracted to the protons in the nucleus by the electromagnetic force. This force binds the electrons inside an electrostatic potential well surrounding the smaller nucleus, which means that an external source of energy is needed for the electron to escape.