Lead-acid Battery
Lead-acid batteries are secondary (rechargeable) batteries that consist of a housing, two lead plates or groups of plates, one of them serving as a positive electrode and the other as a negative electrode, and a filling of 37% sulfuric acid (H2SO4) as electrolyte. The battery contains liquid electrolyte in an unsealed container, requiring it to be kept upright and the area well ventilated to ensure safe dispersal of the hydrogen gas it produces during overcharging. Lead acid batteries typically have coulombic efficiencies of 85% and energy efficiencies in the order of 70%.
Lead and lead dioxide, the active materials on the battery’s plates, react with sulfuric acid in the electrolyte to form lead sulfate. The lead sulfate first forms in a finely divided, amorphous state and easily reverts to lead, lead dioxide, and sulfuric acid when the battery recharges.
Chemistry of Lead-acid Batteries – How it works
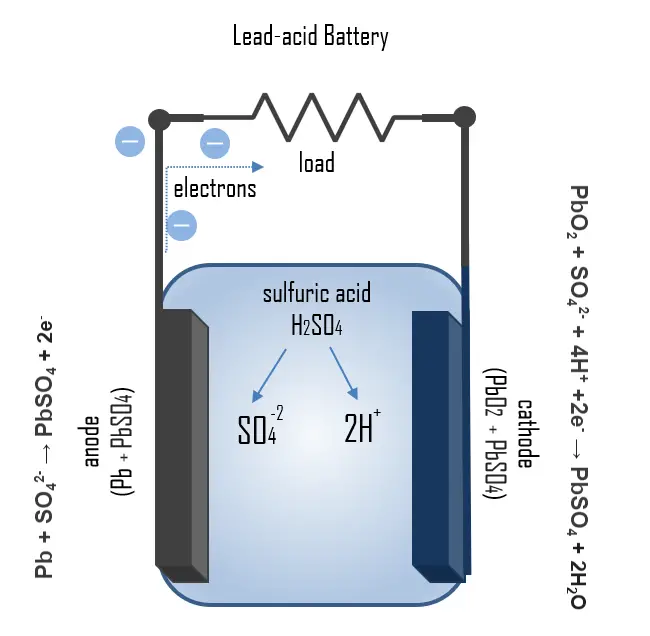
The principle of operation of the lead-acid battery can be illustrated by the chemical processes that take place during charging and discharging. During discharge, the process
Pb + SO42- → PbSO4 + 2e–
takes place at the anode. Lead is oxidized with the electrolyte to lead sulfate, releasing two electrons. Lead sulfate is also formed at the cathode by:
PbO2 + SO42- + 4H+ +2e– → PbSO4 + 2H2O
But in this reaction, a reduction of lead oxide takes place. Formed lead sulfate deposits as a coating on the electrodes and, to some extent also on the bottom of the housing. Since sulfuric acid is utilized during the discharge process, the SoC can be determined by measuring the density of the electrolyte.
During charging, the processes take place in the opposite direction so that the lead sulfate formed during discharging is oxidized to lead and reduced lead oxide, respectively. If the lead sulfate is completely consumed and the changing process is not stopped, electrolysis of the electrolyte begins. Overcharging with high charging voltages generates oxygen and hydrogen gas by electrolysis of water, which bubbles out and is lost. Sealed batteries have catalysts (Pd, Pt) above the vent where oxyhydrogen gas can recombine to water.
The resulting cell voltage can be determined from the galvanic series.
The total voltage of the redox reaction is thus:
E0 = 1.68V – ( – 0.36V) = 2.04V.

