Silver-oxide Battery
A silver-oxide battery is a primary cell using silver oxide as the cathode material, zinc for the anode, and a highly alkaline electrolyte consisting of either sodium hydroxide or potassium hydroxide. They are available in small sizes as button cells. The first letter in the IEC standard system identifies the chemical composition of the battery. S is for silver-oxide battery (as SR516). These cells maintain a nearly constant nominal voltage during discharge until fully depleted. A silver-oxide battery and a zinc-silver battery are different types of batteries.
The open circuit voltage of silver oxide batteries is 1.6 volts. The operating voltage at typical current drains is 1.55 volts or more. A typical silver-oxide battery in the standard SR721SW has about 25 mAh.
Advantages and Disadvantages of Silver-oxide Batteries
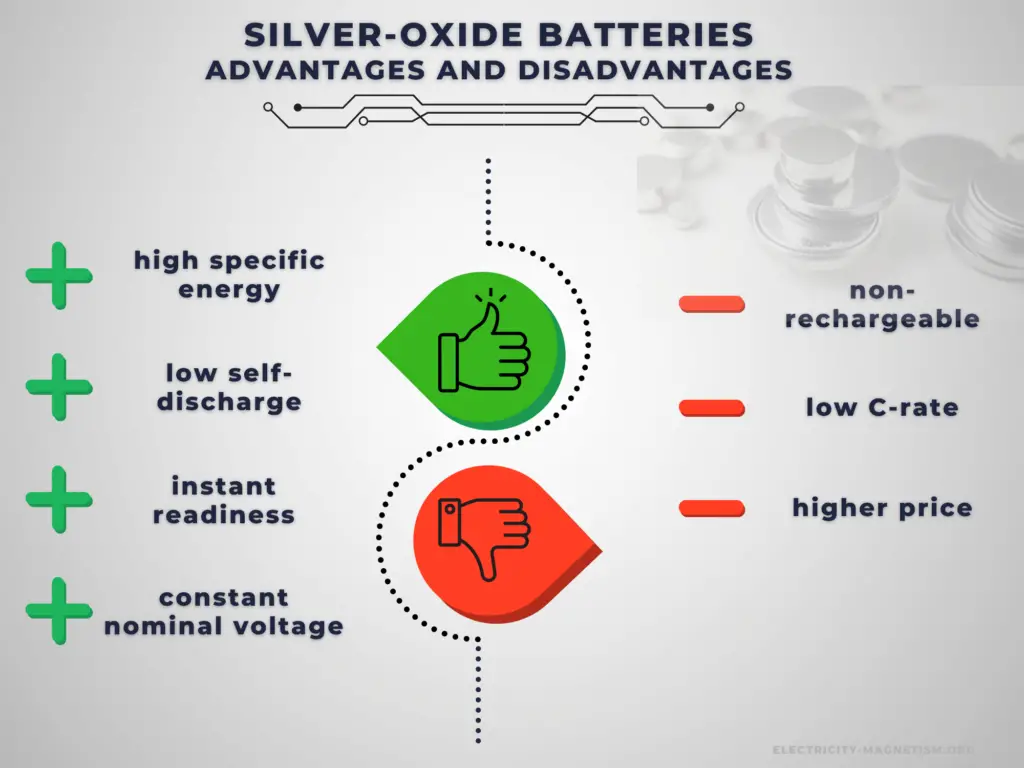
Advantages:
Primary batteries have higher energy density than rechargeable secondary cells. High specific energy, long storage times (low self-discharge), and instant readiness give primary batteries a unique advantage over other power sources. They are usually the best choice for low-drain applications. They can be carried to remote locations and used instantly, even after long storage; they are also readily available and environmentally friendly when disposed.
Disadvantages:
The main disadvantage of primary batteries is that they are non-rechargeable. Another disadvantage is their low C-rate. Even high current types are considered low in comparison to rechargeable batteries. They are also less environment friendly than rechargeable batteries. The application of primary batteries leads to a large amount of waste batteries to be recycled. For large batteries, primary batteries are usually not cost-effective.

