Chemiresistor gas sensors detect target gas molecules by measuring changes in electrical resistance, enabling diverse applications in safety and air quality monitoring.
Chemiresistor Gas Sensors: An Overview
Introduction
Chemiresistor gas sensors are a widely used class of gas detection devices that leverage changes in electrical resistance upon exposure to specific target gas molecules. These sensors play a critical role in a variety of applications, including environmental monitoring, industrial safety, and air quality control. In this article, we will provide an overview of chemiresistor gas sensors, discuss their working principle, and explore the key factors that influence their performance.
Working Principle of Chemiresistor Gas Sensors
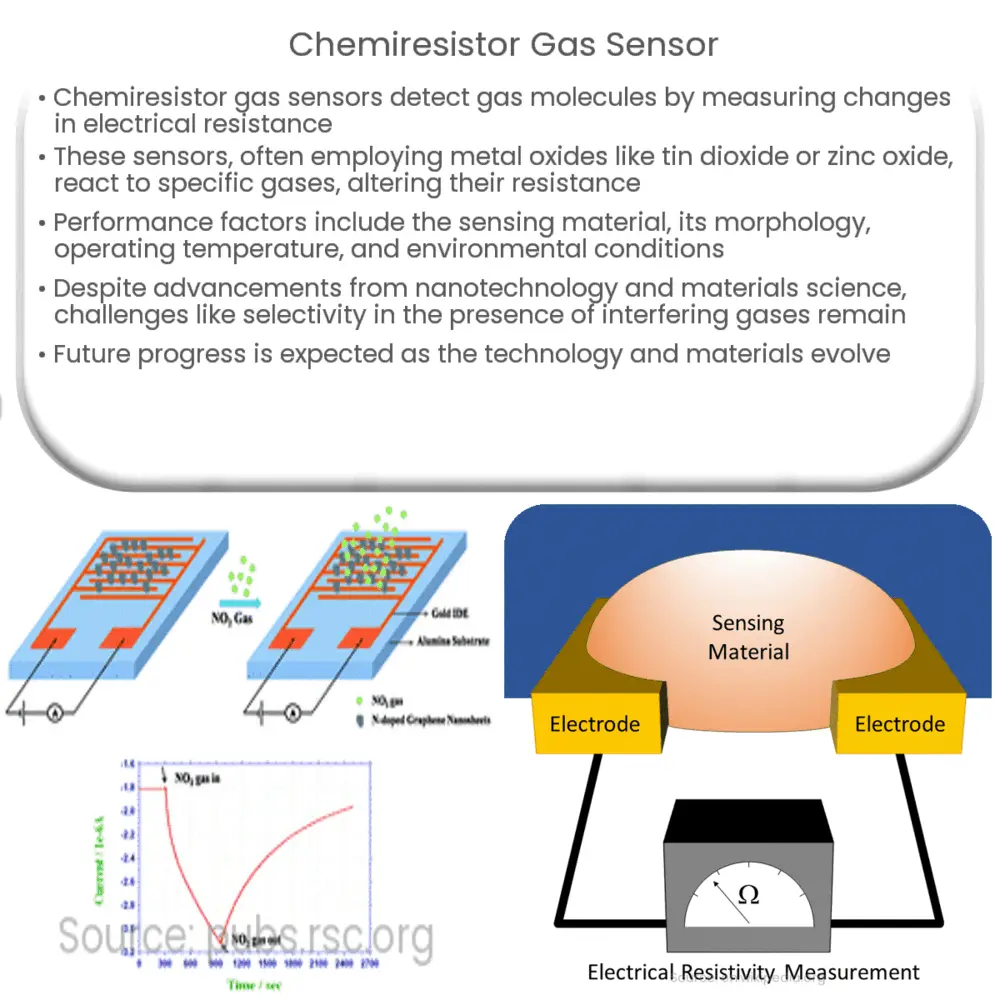
Chemiresistor gas sensors consist of a sensing material, often a metal oxide, whose electrical resistance changes when exposed to specific gas molecules. The sensing material is typically deposited on a substrate, and a pair of electrodes is used to measure the resistance. Upon exposure to the target gas, the sensing material undergoes a chemical reaction, which leads to changes in the electrical resistance. This change in resistance is proportional to the concentration of the target gas in the environment, allowing for quantitative detection.
One of the most common types of chemiresistor gas sensors is based on metal oxide semiconductors (MOS). These sensors typically employ metal oxides like tin dioxide (SnO2), zinc oxide (ZnO), and titanium dioxide (TiO2) as the sensing materials. In the presence of a reducing gas, such as carbon monoxide (CO), the metal oxide undergoes a reduction reaction, which leads to a decrease in its resistance. Conversely, exposure to an oxidizing gas, such as nitrogen dioxide (NO2), results in an increase in resistance due to an oxidation reaction.
Key Factors Affecting Chemiresistor Gas Sensor Performance
The performance of chemiresistor gas sensors can be influenced by a variety of factors, such as the type and morphology of the sensing material, operating temperature, and environmental conditions. We will briefly discuss each of these factors below:
- Sensing Material: The choice of sensing material is crucial for determining the selectivity, sensitivity, and response time of the chemiresistor gas sensor. Different metal oxides exhibit varying affinities for specific gas molecules, which can be exploited to create sensors with tailored selectivity. Additionally, the sensitivity and response time of the sensor can be optimized by selecting materials with appropriate electrical properties.
- Morphology: The morphology of the sensing material can significantly impact the sensor’s performance. Nanostructured materials, such as nanoparticles, nanowires, and thin films, offer higher surface-to-volume ratios compared to their bulk counterparts, which can enhance the sensitivity and response time of the sensor. Furthermore, the porous structure of these materials can facilitate gas diffusion and improve the overall sensing performance.
- Operating Temperature: Chemiresistor gas sensors typically operate at elevated temperatures to facilitate the gas-sensing reactions. The operating temperature can significantly influence the sensitivity, selectivity, and response time of the sensor. Optimal operating temperatures depend on the sensing material and the target gas and can be adjusted by incorporating a heater element into the sensor design.
Advancements and Challenges in Chemiresistor Gas Sensor Technology
Recent advancements in materials science and nanotechnology have led to the development of novel sensing materials and morphologies, resulting in improved chemiresistor gas sensor performance. These advancements include the use of hybrid materials, such as metal oxide composites and conductive polymers, as well as the development of hierarchical nanostructures that enhance gas-sensing properties. Furthermore, the integration of chemiresistor gas sensors with microelectromechanical systems (MEMS) has enabled the miniaturization and low-power operation of these devices.
Despite these advancements, challenges remain in the development of chemiresistor gas sensors. Improving the selectivity of these sensors in the presence of interfering gases is a critical issue, as it directly affects their applicability in real-world situations. Additionally, the long-term stability and repeatability of chemiresistor gas sensors are essential for their adoption in continuous monitoring applications. Addressing these challenges necessitates further research and innovation in the field of gas sensing materials and sensor design.
Conclusion
Chemiresistor gas sensors are a versatile and widely-used technology for detecting and monitoring gas concentrations in various applications. By understanding the fundamental principles of chemiresistive sensing and the factors that influence sensor performance, researchers and engineers can develop more advanced and reliable gas sensors for a range of applications, from environmental monitoring to industrial safety. As materials science and nanotechnology continue to advance, it is expected that chemiresistor gas sensor technology will also see significant improvements, leading to better selectivity, sensitivity, and stability in the future.

