Explore the Kelvin Water Dropper’s principles, applications, innovations, limitations, and its vital educational role in understanding electrostatics.
Introduction to Kelvin Water Dropper
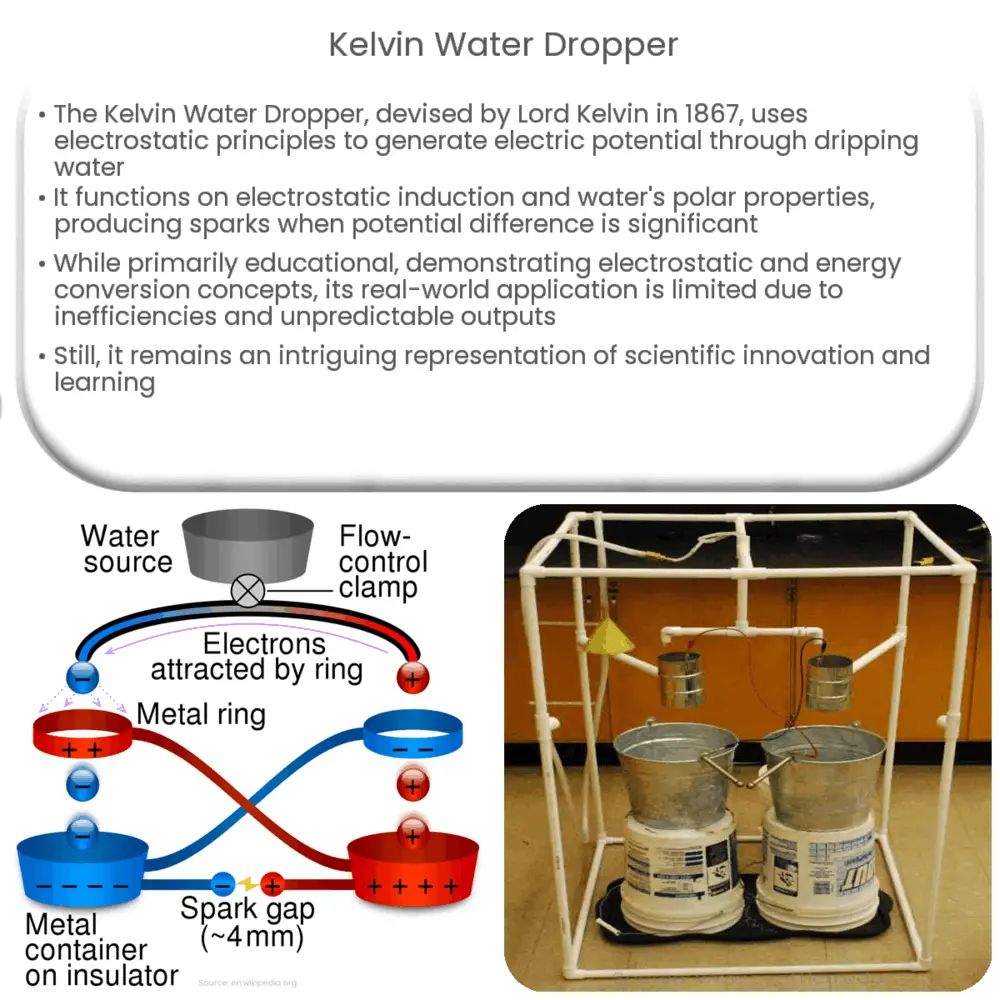
The Kelvin Water Dropper, also known as the Kelvin Hydroelectric Generator, is an intriguing device that leverages the principles of electrostatics to generate a potential difference by simply dripping water. Named after Lord Kelvin who first devised the apparatus in 1867, it showcases how electrostatic charges can be manipulated to create a substantial electric potential.
Working Principle of Kelvin Water Dropper
The underlying physics that drives the operation of the Kelvin Water Dropper is essentially electrostatic induction and the inherent properties of water, especially its polar nature. Its ingenious design features two pairs of containers where one pair of containers serves as a reservoir for water, while the other pair functions as a receiver. The pairs of containers are cross-connected through metal wires, with each reservoir having a small hole at the bottom to allow water to drip out.
- The process begins with the water in the reservoirs dripping out through the small holes. As the droplets pass through air, they become charged due to the electrostatic charge in the environment.
- The droplets land in the receiving containers, effectively transferring the charge. Because of the metal wires connecting the reservoirs to the receivers, the charge is transferred back to the water still in the reservoirs.
- This causes the subsequent water droplets to be charged in the opposite polarity, enhancing the charge difference between the two receivers.
- When the potential difference between the receivers becomes sufficiently large, a spark will jump across the gap, discharging the system.
Applications of Kelvin Water Dropper
While the Kelvin Water Dropper may seem like a simple science experiment, it possesses considerable pedagogical value, often serving as an instructional tool in physics classrooms to illustrate the principles of electrostatic induction and electric potential. Not only does it demonstrate the transfer of charge, but it also vividly displays the conversion of gravitational potential energy into electrical energy.
Moreover, the Kelvin Water Dropper underscores the concept of charge conservation and brings to life the abstract concept of potential difference in a highly visual and interactive manner. Though it does not have major practical applications in modern energy production due to its inefficiency, the device remains an impressive demonstration of the fundamental principles of electrostatics.
Additionally, with some modifications and upscaling, the Kelvin Water Dropper could be used as a low-power generator in situations where only minimal power is needed, and a continuous water source is available. However, such use cases are niche and often overshadowed by more efficient energy conversion technologies.
Further Explorations and Innovations
Though the Kelvin Water Dropper has limited practical utility in its original form, it does provide a basis for further innovations and explorations in the realm of energy generation and conversion. The simplicity of the device makes it ripe for tinkering and adaptation, particularly in educational settings where the students are encouraged to experiment and invent.
Over the years, several variations of the Kelvin Water Dropper have been developed. Some of these modifications include the use of alternative fluids with different conductivity properties, altering the structure and design to enhance efficiency, and even using the device as a component in larger systems for demonstrating or studying different physical phenomena.
Limitations of the Kelvin Water Dropper
Despite its pedagogical usefulness and conceptual beauty, the Kelvin Water Dropper is not without its limitations. The device is inherently inefficient, with a significant amount of the gravitational potential energy of the water being lost as heat and sound during the discharge process. Furthermore, the device relies heavily on the environmental conditions – the presence of ambient electrostatic charge, air pressure, and humidity can greatly influence the dropper’s performance.
Another major limitation is the necessity of a continuous water supply. In real-world applications, such a requirement can often be impractical. Lastly, the electrostatic potential created by the device is largely uncontrolled and can vary wildly, making it unsuitable for applications that require stable and predictable power output.
Conclusion
In conclusion, the Kelvin Water Dropper is a fascinating device that beautifully exemplifies the principles of electrostatics and energy conversion. While its practical applications are limited, the device holds substantial pedagogical value, often being used in educational settings to illustrate complex physical principles. The device’s simple design and operation also make it an excellent platform for further explorations and innovations. Despite its limitations, the Kelvin Water Dropper stands as a testament to the ingenuity of its creator and continues to inspire and educate future generations of scientists and engineers.

