Temperature affects conductivity differently: conductors’ conductivity decreases, insulators and semiconductors’ conductivity increases, and superconductors vary.
Temperature and Electrical Conductivity
Temperature plays a crucial role in determining the electrical conductivity of materials. Different materials exhibit varying conductivity behavior in response to changes in temperature. To understand the impact of temperature on conductivity, it is essential to classify materials into two primary categories: conductors and insulators.
Conductors
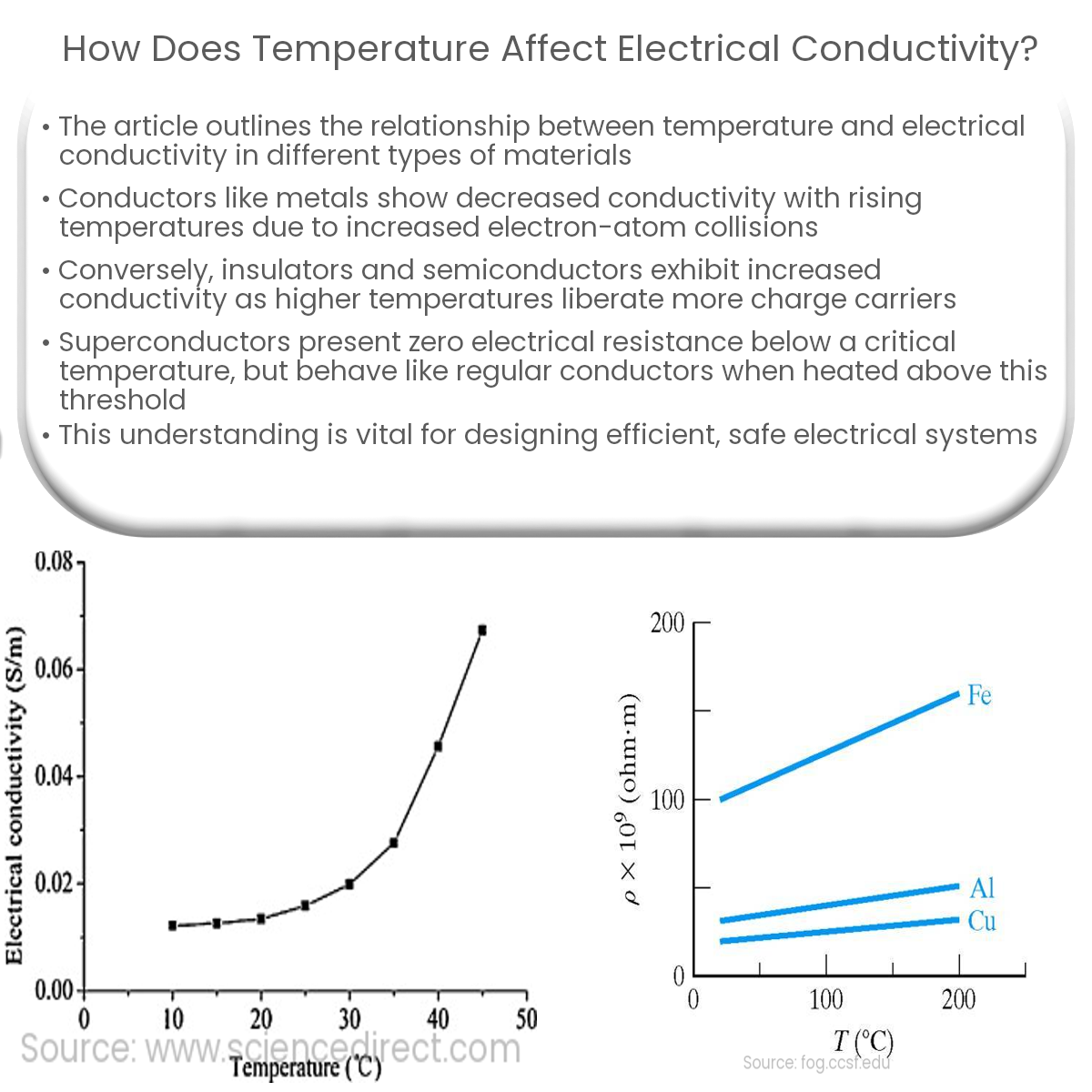
Conductors, such as metals and alloys, allow the flow of electric current due to the presence of free electrons in their atomic structure. As the temperature increases, the atoms in conductive materials vibrate more, creating a higher degree of lattice disorder. This increased vibration causes a greater number of collisions between free electrons and atoms, which results in increased resistance and decreased electrical conductivity.
Insulators and Semiconductors
Insulators, such as plastics, glass, and ceramics, generally exhibit low electrical conductivity. Semiconductors, on the other hand, have intermediate conductivity that can be manipulated by adding impurities or dopants. Both insulators and semiconductors display an opposite trend to conductors when subjected to temperature changes.
As the temperature rises, insulators and semiconductors experience an increase in electrical conductivity. This occurs because higher temperatures provide enough energy to liberate more charge carriers (electrons and holes) from their atomic structures, resulting in a larger number of mobile charge carriers available for conduction.
Superconductors
Superconductors are a unique class of materials that exhibit zero electrical resistance when cooled below a certain critical temperature. This results in perfect electrical conductivity, allowing electric current to flow without any energy loss. However, when the temperature rises above the critical temperature, superconductors lose their superconducting properties and revert to behaving like ordinary conductors, with resistance and reduced conductivity.
Conclusion
The relationship between temperature and electrical conductivity varies depending on the type of material. While conductors experience a decrease in conductivity with increasing temperature, insulators and semiconductors typically show the opposite trend. Superconductors demonstrate perfect conductivity below a critical temperature, losing this property when the temperature rises. Understanding the temperature-conductivity relationship is essential for designing and operating electrical systems and devices efficiently and safely.