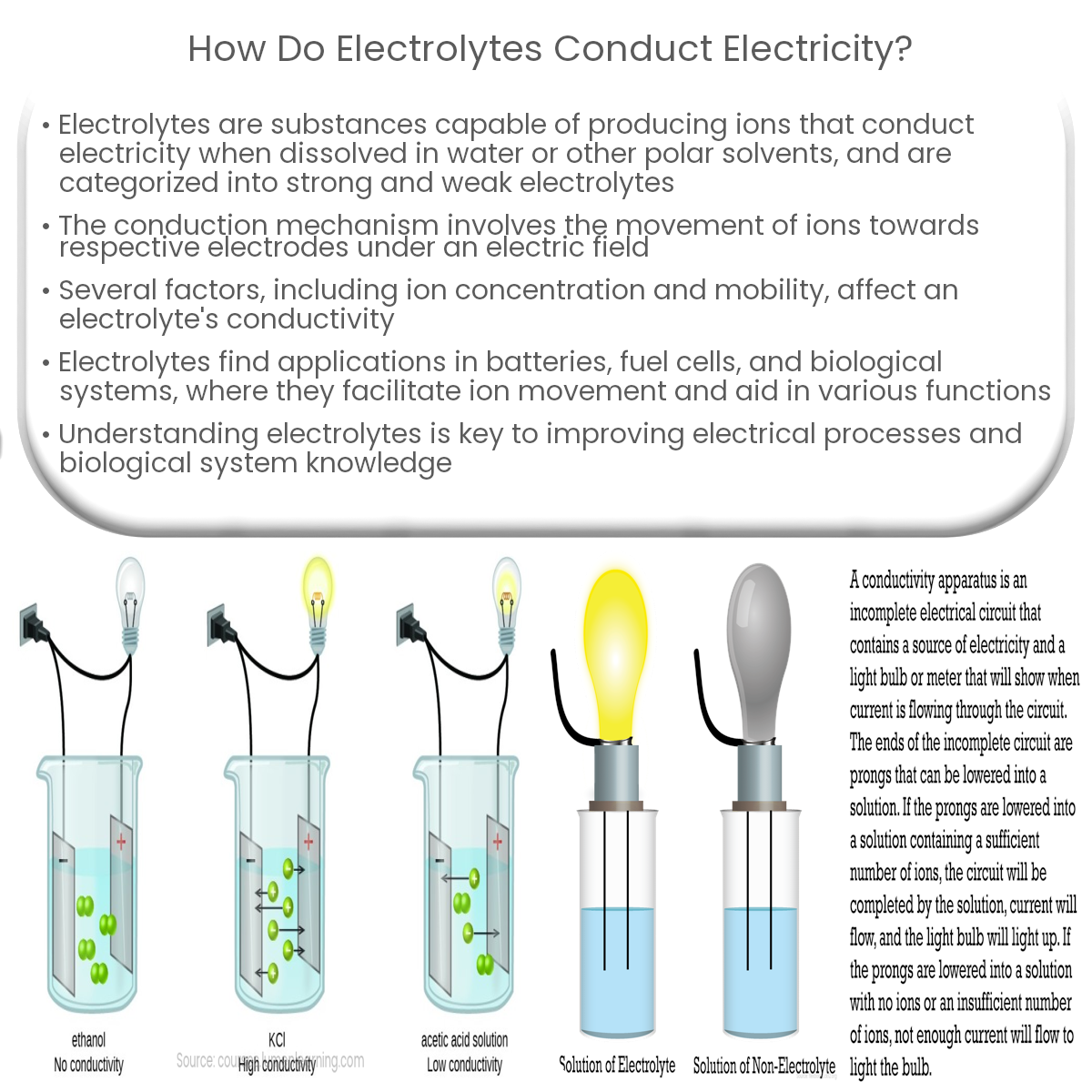
Electrolytes conduct electricity by dissociating into ions in a solution, which then move under an electric field, allowing current to flow.
Electrolytes: Conductors of Electricity
Electrolytes are substances that, when dissolved in water or other polar solvents, produce ions capable of conducting electricity. They play a vital role in numerous applications, from batteries and fuel cells to biological systems.
Types of Electrolytes
Electrolytes can be categorized into two main groups:
Electrical Conduction Mechanism
Electrolytic conduction relies on the movement of ions within a solution. When an electrolyte is dissolved in water, the resulting solution contains free positive and negative ions. When an electric field is applied to the solution, ions with opposite charges move towards the respective electrodes (anode and cathode), allowing current to flow through the solution.
The overall conductivity of an electrolyte depends on factors such as the concentration of ions, the mobility of the ions, and the temperature of the solution. Electrolytes with a higher concentration of ions and greater ion mobility generally exhibit better conductivity.
Applications
Electrolytes find use in a variety of applications:
Understanding the properties and behavior of electrolytes is essential for improving the efficiency of various electrical and electrochemical processes, as well as for enhancing our knowledge of biological systems and their functions.