Ions are formed through chemical reactions, ionization, and electrolysis, resulting from electron gain or loss in atoms or molecules.
Introduction to Ion Formation
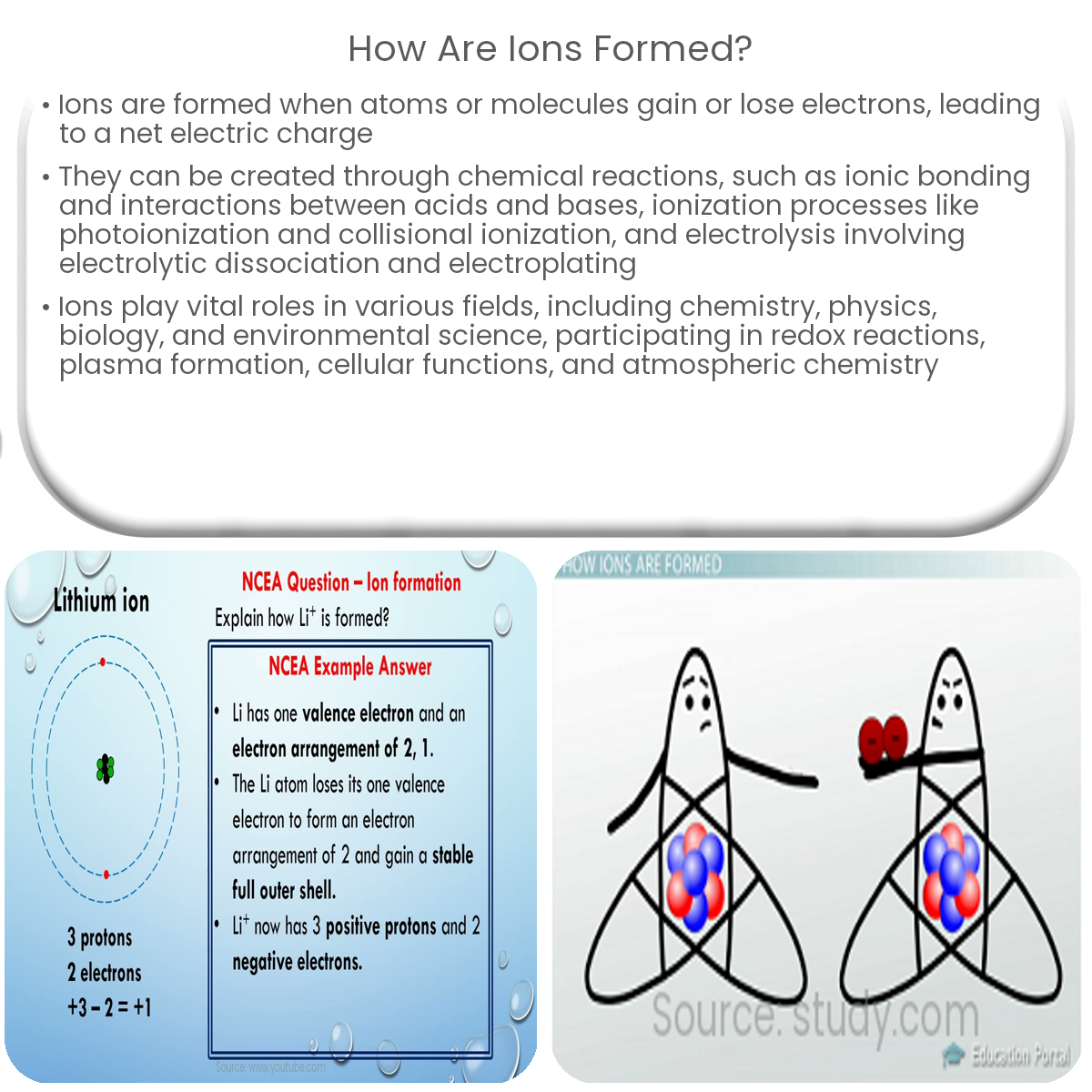
Ions are formed when atoms or molecules gain or lose electrons, resulting in a net electric charge. Several processes can lead to the formation of ions, including chemical reactions, ionization, and electrolysis. This article explores these ion-forming processes in detail.
Chemical Reactions
During chemical reactions, atoms can exchange electrons to achieve more stable electron configurations:
Ionic Compounds: Atoms with different electronegativities can undergo ionic bonding, in which one or more electrons are transferred from one atom to another. The resulting charged atoms, or ions, attract each other and form an ionic compound. Metals often lose electrons to form cations, while nonmetals gain electrons to form anions.
Acids and Bases: In aqueous solutions, acids release hydrogen cations (H+), while bases release hydroxide anions (OH–).
Ionization
Ionization involves the removal or addition of electrons from/to atoms or molecules by high-energy particles or radiation:
Photoionization: When a high-energy photon interacts with an atom or molecule, it can transfer enough energy to eject an electron, creating a positively charged ion.
Collisional Ionization: High-energy particles, such as electrons or ions, can collide with atoms or molecules and transfer sufficient energy to remove an electron, creating an ion.
Field Ionization: Extremely strong electric fields can cause atoms or molecules to lose electrons, forming ions.
Electrolysis
Electrolysis is the process of driving a non-spontaneous redox reaction using an electric current. During electrolysis, ions can form as the applied voltage causes dissociation and migration toward the electrodes:
Electrolytic Dissociation: When an electric current is applied to a solution or a molten ionic compound, ions dissociate and migrate toward the electrodes.
Electroplating: In an electrolytic cell, metal ions can be reduced at the cathode, depositing a thin layer of metal onto an object.
Electrolytic Production: Electrolysis can produce chemical compounds, such as chlorine and hydrogen gas, through the oxidation and reduction of ions at the electrodes.
Role of Ions in Various Fields
Ions formed through these processes play crucial roles in numerous scientific and technological fields, including:
Chemistry: Ions participate in redox reactions, ionic bonding, and solubility processes.
Physics: Ionized gases, or plasmas, are crucial in fusion research, lighting, and ion propulsion.
Biology: Ions are essential for cellular functions, enzyme activity, and electrolyte balance.
Environment: Ion formation affects atmospheric chemistry, such as ozone formation and destruction.