30-second summary
Nickel Metal Hydride Battery
A nickel metal hydride battery, NiMH, is a rechargeable battery with a positive electrode made of nickel hydroxide and a negative electrode made of a metal hydride (a hydrogen-absorbing alloy).
Compared to the NiCd battery, the NiMH provides 40 percent higher specific energy resulting in about two times higher capacity.
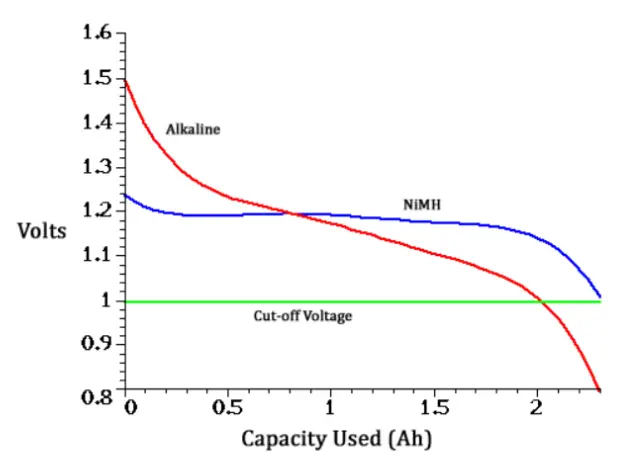
Compared to alkaline batteries, the internal resistance of NiMH batteries is much lower. Because of this, they have the advantage that a higher voltage can be maintained under high load.
The overall reaction during discharge is:
NiO(OH) + MH → Ni(OH)2 + M

Nickel Metal Hydride Battery
A nickel metal hydride battery, NiMH, is a rechargeable battery with a positive electrode made of nickel hydroxide and a negative electrode made of a metal hydride (a hydrogen-absorbing alloy). The NiMH battery was commercially introduced in 1989 and was mainly used as a power source in portable personal computers. Since then, the NiMH battery system has become very popular in electric hybrid vehicles and makes up 10% of the total market for rechargeable batteries. Compared to the NiCd battery, the NiMH provides 40 percent higher specific energy resulting in about two times higher capacity. NiMH batteries are also less affected by voltage depression, but the main advantage is the absence of toxic cadmium. The memory effect of NiMH batteries is much less than nickel-cadmium batteries.
Compared to alkaline batteries, the internal resistance of NiMH batteries is much lower. Because of this, they have the advantage that a higher voltage can be maintained under high load.
With high specific energy (up to 100Wh/kg) and energy density (double of lead-acid and 40% more than nickel-cadmium), high cycle life, low cost, recyclability (cadmium is hazardous), and other qualities made it the most used battery and still nowadays is one of the most used. Its applications are much diverse: battery cells AA or AAA, cameras, old mobile phones, electric razors, medical instruments and equipment, and high-power static applications. Compared to the lead-acid battery, the drawback is that it is more expensive than lead acid per kWh.
NiMH batteries are (re)charged by applying electric current, which reverses the chemical reactions that occur during discharge/use. Devices to supply the appropriate current are called chargers. The electronics in charging systems and control circuits for NiMHs are simple and inexpensive, and the battery is considered safe. The NiMH battery also has high self-discharge and can lose up to 20 % of its charge during the first 24 hours and thereafter 10 % per month.
Like NiCd batteries, they have a nominal voltage of 1.2V per cell with a typical end-of-discharge voltage of 1V.
The total voltage of the redox reaction is E0 = 0.49V – ( – 0.83V) = 1.32V.
Nevertheless, they are widely used as a replacement for alkaline batteries, which have 1.5V nominal voltage per cell, in many applications and are commonly found in standard battery designs. In some applications, however, the lower nominal voltage can be a disadvantage. For example, unregulated lights that are designed for 1.5V operation tend to shine less brightly.
Chemistry of Nickel Metal Hydride Battery – How it works
A typical NiMH cell contains:
- Cathode: a nickel(III) oxide-hydroxide positive electrode plate
- Anode: a metal hydride (a hydrogen-absorbing alloy)
- Separator
- Electrolyte: an alkaline electrolyte (potassium hydroxide).
The metal M in the negative electrode of a NiMH cell is an intermetallic compound. Many different compounds have been developed for this application. The research on materials for negative electrodes for the Ni/MH battery has involved several alloy families: AB5, AB2, and AB. In this notation, A represents a metallic element with a strong affinity to hydrogen (typically rare-earth element like lanthanum, cerium, neodymium, or praseodymium), whereas B is a metallic element with weak hydrogen affinity (typically transition metal like nickel, cobalt, manganese, or aluminum). Today the hydride-forming materials based on the AB5 intermetallics are the most versatile and commercially important family of reversible hydriding alloys. The optimal composition for electrode purposes has been found for an alloy with the following composition:
MmNi3.55Mn0.4Al0.3Co0.75
where Mm is a Mischmetal, which is an alloy of rare-earth elements. It is also called cerium mischmetal or rare-earth mischmetal. A typical composition includes approximately 55% cerium, 25% lanthanum, and 15~18% neodymium, with traces of other rare earth metals.
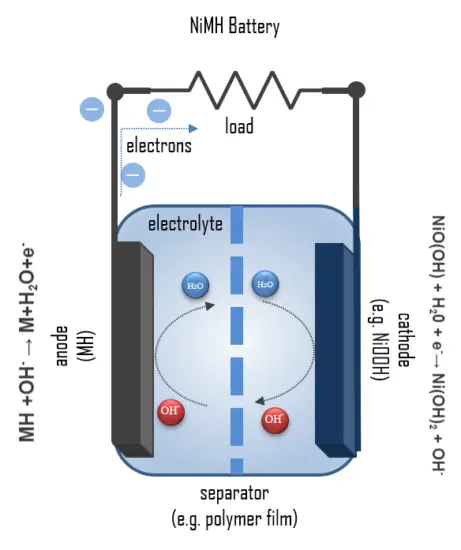
The layer structure of a NiMH round cell can be seen in the figure. An outer perforated foil serves as a carrier for the metal hydride powder, which forms the negative electrode. During discharge, the hydrogen bound in the metal hydride MH is oxidized to a proton, and metal of 0 oxidation state is formed:
MH +OH– → M+H2O+e–
The resulting protons react with the OH– hydroxide ions of the KOH solution to form water. The redox potential is approximately -0.83 V. The separator holds the electrolyte, a 20% KOH solution, and prevents direct contact with the positive electrode. The cathode consists of a sheet of nickel hydroxide and nickel oxide hydrate. Here, nickel of oxidation state +III is reduced to nickel of oxidation state +II in the reaction:
NiO(OH) + H20 + e–→ Ni(OH)2 + OH–
The overall reaction during discharge is:
NiO(OH) + MH → Ni(OH)2 + M
During this process, free electrons are bound so that this pole becomes the positive electrode. The redox voltage of the reduction is approximately 0.49 V. The total voltage of the redox reaction is thus E0 = 0.49V – ( – 0.83V) = 1.32V.
The specific energy of a NiMH cell is about 80 Wh/kg, which is almost as high as that of an alkaline cell and more than twice as high as that of a NiCd battery. NiMH batteries are sensitive to overcharging, overheating, incorrect polarity, and also to deep discharge.
Advantages and Disadvantages of Nickel Metal Hydride Batteries
Advantages:
The main advantage of rechargeable cells is that they may be recharged after discharge. Therefore, rechargeable batteries are more environmentally friendly than primary batteries. Not only can they be used repeatedly, but they generate less waste over the long term. This is particularly true in the case of power-intensive devices, which consume batteries at an increased rate. Another very important advantage is a high C-rate. Rechargeable cells have better power output capabilities compared to primary cells and are used for high-power applications.
There are several specific advantages to NiMH batteries.
- delivers high current output
- rapid recharge capability
- less expensive than lithium-based battery systems
Disadvantages:
Battery price is one of the challenging factors in choosing the right rechargeable battery for your device or applications. It greatly affects the decision of the buyer. Rechargeable batteries have higher initial costs than their primary counterparts. Another important disadvantage is their self-discharge. In low-drain applications, the service life is more important, and the self-discharge characteristics of a rechargeable battery mean that they are less suitable for use as the primary energy source.
There are several specific disadvantages to NiMH batteries.
- not suitable for shallow cycling
- lower specific energy and specific power than lithium-based battery systems
Characteristics of Nickel Metal Hydride Batteries
To compare and understand the capability of each battery, some important parameters are characteristic of each battery, also within a type of battery. These parameters are a reference when a battery is needed, and specific qualities are required since batteries are used in all types of devices and for infinite purposes.
Cell Voltage
The voltage of electric batteries is created by the potential difference of the materials that compose the positive and negative electrodes in the electrochemical reaction.
A common open circuit voltage for NiMH batteries (e.g. AAA and AA) is 1.2V.
Cut-off Voltage
The cut-off voltage is the minimum allowable voltage. It is this voltage that generally defines the “empty” state of the battery.
When testing the capacity of a NiMH or NiCd battery, a cut-off voltage of 1.0 V per cell is normally used, whereas 0.9 V is normally used as the cut-off voltage of an alkaline cell.
Capacity
The coulometric capacity is the total Amp-hours available when the battery is discharged at a certain discharge current from 100% SOC to the cut-off voltage.
NiMH AA batteries feature a nominal voltage of 1.2 volts and an average capacity of 2000-2700 mAh.
C-rate of Battery
C-rate is used to express how fast a battery is discharged or charged relative to its maximum capacity. It has units h−1. A 1C rate means that the discharge current will discharge the entire battery in 1 hour.
Typically, high C-rate NiMH batteries can be charged at 1C and be fully charged in just over an hour.
Self-discharge
Batteries gradually self-discharge even if not connected and delivering current. This is due to non-current-producing “side” chemical reactions that occur within the cell even when no load is applied.
The NiMH battery also has high self-discharge and can lose up to 20 % of its charge during the first 24 hours and thereafter 10 % per month.
Degradation
Some degradation of rechargeable batteries occurs on each charge-discharge cycle. Degradation usually occurs because electrolyte migrates away from the electrodes or because active material detaches from the electrodes.
The cycle life for NiMH batteries is typically 700-1,000 life cycles.
Depth of Discharge
Depth of discharge is a measure of how much energy has been withdrawn from a battery and is expressed as a percentage of full capacity. For example, a 100 Ah battery from which 40 Ah has been withdrawn has undergone a 40% depth of discharge (DOD).
Why are alkaline batteries (AAA or AA) made to be 1.5V while rechargeables are 1.2V?
In general, batteries convert stored chemical energy into electrical energy through an electrochemical process. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits.
- Primary (single-use or alkaline) batteries use cells that have 1.5V open circuit voltage when fresh.
- Secondary (rechargeable) batteries use cells from NiMh or NiCd, which have 1.2V open circuit voltage.
In practice, alkaline batteries and rechargeable batteries can be used interchangeably in sets. They have only different voltage characteristics. It is given by their different chemistry. Primary cells gradually drop in voltage from use. They start at 1.5 volts, drop to 1.2 and continue to 1.0 where the appliance stops working. Secondary cells operate more uniformly, even with only 1.2 volts. They have flat discharge where they pretty much stay at 1.2 volts until depleted and then drop off very quickly to below 1.0 volts.
Since electronic devices are usually made to run from cell voltages of 1.0 to 1.5 volts, alkaline batteries and rechargeable batteries perform similarly. In fact, it’s generally considered that secondary 1.2 V cells work better than alkalines having lower output impedance and more consistent voltage from start to finish of a charge.
Other Types of Batteries
The following list summarizes notable electric battery types composed of one or more electrochemical cells. Four lists are provided in the table. The first list is a battery classification by size and format. Then, the primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. The final list is a list of different battery voltages.
Sizes
- AA battery
- AAA battery
- AAAA battery
- C battery
- D battery
- cr1220 battery
- cr1620 battery
- cr1632 battery
- cr1616 battery
- cr2016 battery
- cr2032 battery
- cr2025 battery
- cr2430 battery
- cr2450 battery
- cr123 battery
- cr2 battery
- cr132a battery
- lr1130 battery
- lr41 battery
- lr44 battery
- A23 battery
- a13 battery
- 18650 battery
- 21700 battery
Frequently asked questions
There are two basic types of batteries: primary and secondary. Primary batteries are “single use” and cannot be recharged. Dry cells and (most) alkaline batteries are examples of primary batteries. The second type is rechargeable and is called a secondary battery.
NiMH batteries are common rechargeable batteries of AA or AAA format. They can also be used in electric razors, toothbrushes, cameras, camcorders, mobile phones, pagers, medical instruments/equipment, and numerous other applications.
There are several specific advantages to NiMH batteries. They can deliver high current output, have rapid recharge capability, and are less expensive than lithium-based battery systems. On the other hand, NiMH batteries are not suitable for shallow cycling and offer lower specific energy and specific power than lithium-based battery systems.