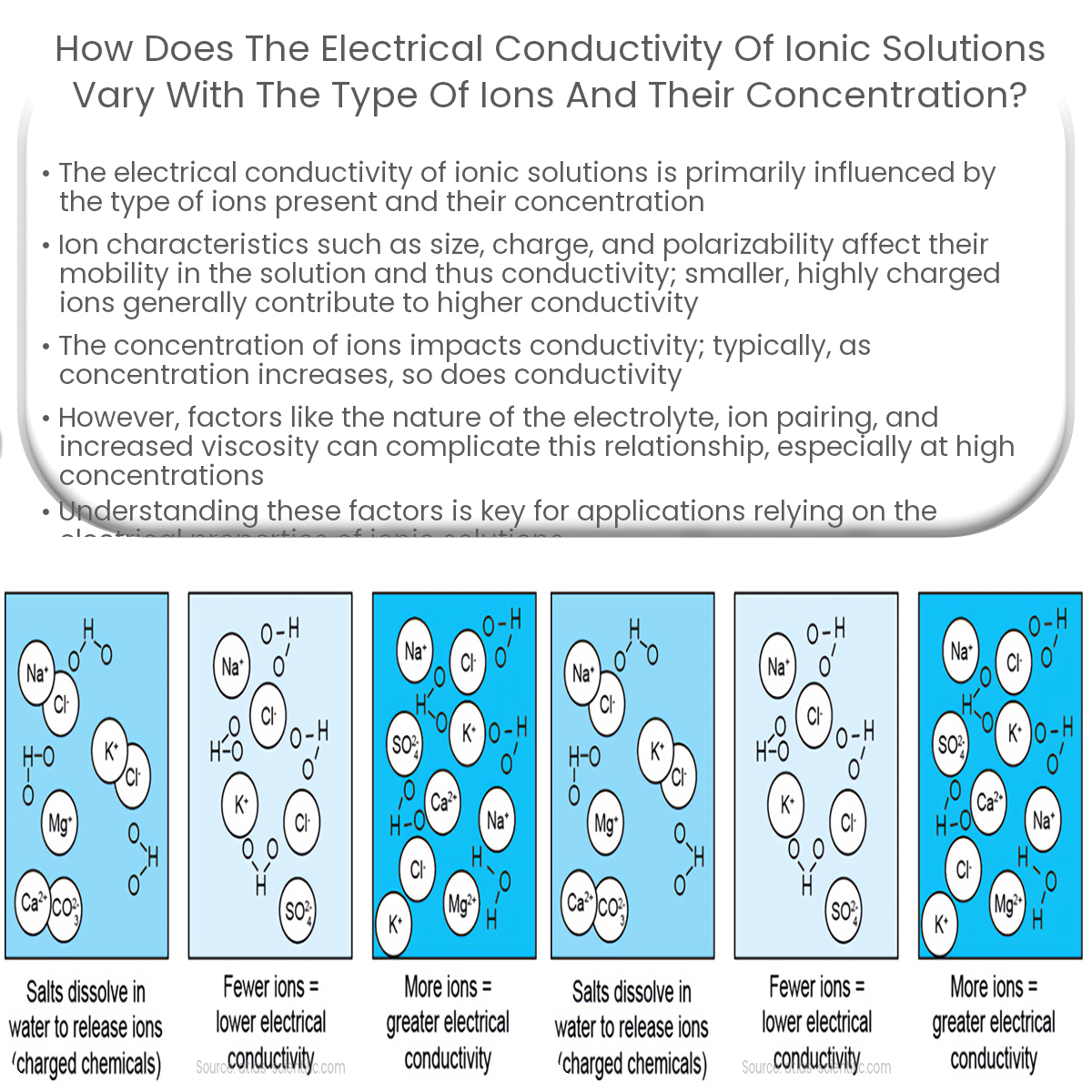
Electrical conductivity in ionic solutions depends on ion type, charge, mobility, concentration, electrolyte nature, ion pairing, and solution viscosity.
Electrical Conductivity of Ionic Solutions
Electrical conductivity in ionic solutions depends on several factors, including the type of ions present and their concentration. In this article, we will explore how these factors affect the conductivity of an ionic solution.
Type of Ions
Electrical conductivity is mainly determined by the charge and mobility of ions in the solution. Different ions have different charges and mobilities, which leads to variations in conductivity. Some key factors influencing ion mobility include:
- Size: Smaller ions move faster in the solution, contributing to higher conductivity.
- Charge: Ions with higher charges experience stronger electrostatic interactions, which affects their mobility and, in turn, conductivity.
- Polarizability: Highly polarizable ions interact more strongly with the surrounding solvent, reducing their mobility and conductivity.
These factors combine to create unique conductivity profiles for each type of ion in the solution.
Concentration of Ions
The concentration of ions in the solution also plays a critical role in determining electrical conductivity. Generally, as the concentration of ions increases, so does conductivity, since there are more charge carriers available to facilitate the movement of electricity. However, this relationship is not always linear and can be influenced by the following factors:
- Electrolyte nature: Strong electrolytes, like salts, fully dissociate into ions, while weak electrolytes, like weak acids and bases, only partially dissociate. Thus, strong electrolytes typically exhibit higher conductivity.
- Ion pairing: At higher concentrations, ions can form temporary pairs or clusters, reducing the effective number of charge carriers and conductivity.
- Viscosity: Increased ion concentration can lead to increased solution viscosity, impeding ion mobility and reducing conductivity.
At very low concentrations, the conductivity of an ionic solution is directly proportional to the concentration of ions. However, at higher concentrations, the relationship becomes more complex, as the aforementioned factors come into play.
Conclusion
The electrical conductivity of ionic solutions depends on the type of ions and their concentration. Factors like ion size, charge, polarizability, electrolyte nature, ion pairing, and viscosity all contribute to the overall conductivity of the solution. Understanding these factors is crucial for designing and optimizing applications that rely on the electrical properties of ionic solutions.